Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An archaic HLA class I receptor allele diversifies natural killer cell-driven immunity in First Nations peoples of Oceania
Cell ( IF 45.5 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.cell.2024.10.005 Liyen Loh, Philippa M. Saunders, Camilla Faoro, Neus Font-Porterias, Neda Nemat-Gorgani, Genelle F. Harrison, Suraju Sadeeq, Luca Hensen, Shu Cheng Wong, Jacqueline Widjaja, E. Bridie Clemens, Shiying Zhu, Katherine M. Kichula, Sudan Tao, Faming Zhu, Gonzalo Montero-Martin, Marcelo Fernandez-Vina, Lisbeth A. Guethlein, Julian P. Vivian, Jane Davies, Paul J. Norman
Cell ( IF 45.5 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.cell.2024.10.005 Liyen Loh, Philippa M. Saunders, Camilla Faoro, Neus Font-Porterias, Neda Nemat-Gorgani, Genelle F. Harrison, Suraju Sadeeq, Luca Hensen, Shu Cheng Wong, Jacqueline Widjaja, E. Bridie Clemens, Shiying Zhu, Katherine M. Kichula, Sudan Tao, Faming Zhu, Gonzalo Montero-Martin, Marcelo Fernandez-Vina, Lisbeth A. Guethlein, Julian P. Vivian, Jane Davies, Paul J. Norman
|
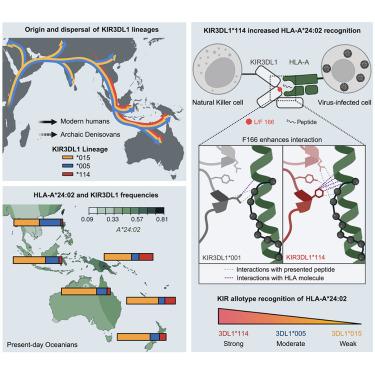
Genetic variation in host immunity impacts the disproportionate burden of infectious diseases that can be experienced by First Nations peoples. Polymorphic human leukocyte antigen (HLA) class I and killer cell immunoglobulin-like receptors (KIRs) are key regulators of natural killer (NK) cells, which mediate early infection control. How this variation impacts their responses across populations is unclear. We show that HLA-A∗24:02 became the dominant ligand for inhibitory KIR3DL1 in First Nations peoples across Oceania, through positive natural selection. We identify KIR3DL1∗114, widespread across and unique to Oceania, as an allele lineage derived from archaic humans. KIR3DL1∗114+NK cells from First Nations Australian donors are inhibited through binding HLA-A∗24:02. The KIR3DL1∗114 lineage is defined by phenylalanine at residue 166. Structural and binding studies show phenylalanine 166 forms multiple unique contacts with HLA-peptide complexes, increasing both affinity and specificity. Accordingly, assessing immunogenetic variation and the functional implications for immunity are fundamental toward understanding population-based disease associations.
中文翻译:

一种古老的 HLA I 类受体等位基因使大洋洲原住民的自然杀伤细胞驱动免疫多样化
宿主免疫力的遗传变异影响了原住民可能经历的不成比例的传染病负担。多态性人类白细胞抗原 (HLA) I 类和杀伤细胞免疫球蛋白样受体 (KIR) 是自然杀伤 (NK) 细胞的关键调节因子,可介导早期感染控制。这种差异如何影响它们在人群中的反应尚不清楚。我们表明,HLA-A∗24:02 通过正自然选择成为大洋洲原住民抑制KIR3DL1的主要配体。我们将 KIR3DL1∗114 确定为源自古人类的等位基因谱系,该基因在大洋洲广泛存在且独一无二。来自澳大利亚原住民供体的 KIR3DL1∗114+NK 细胞通过结合 HLA-A∗24:02 被抑制。KIR3DL1∗114 谱系由残基 166 处的苯丙氨酸定义。结构和结合研究表明,苯丙氨酸 166 与 HLA 肽复合物形成多个独特的接触,从而提高了亲和力和特异性。因此,评估免疫遗传变异和免疫功能意义对于理解基于人群的疾病关联至关重要。
更新日期:2024-10-29
中文翻译:

一种古老的 HLA I 类受体等位基因使大洋洲原住民的自然杀伤细胞驱动免疫多样化
宿主免疫力的遗传变异影响了原住民可能经历的不成比例的传染病负担。多态性人类白细胞抗原 (HLA) I 类和杀伤细胞免疫球蛋白样受体 (KIR) 是自然杀伤 (NK) 细胞的关键调节因子,可介导早期感染控制。这种差异如何影响它们在人群中的反应尚不清楚。我们表明,HLA-A∗24:02 通过正自然选择成为大洋洲原住民抑制KIR3DL1的主要配体。我们将 KIR3DL1∗114 确定为源自古人类的等位基因谱系,该基因在大洋洲广泛存在且独一无二。来自澳大利亚原住民供体的 KIR3DL1∗114+NK 细胞通过结合 HLA-A∗24:02 被抑制。KIR3DL1∗114 谱系由残基 166 处的苯丙氨酸定义。结构和结合研究表明,苯丙氨酸 166 与 HLA 肽复合物形成多个独特的接触,从而提高了亲和力和特异性。因此,评估免疫遗传变异和免疫功能意义对于理解基于人群的疾病关联至关重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号