当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-catalyzed thiolation of C(sp3)–H with sulfonyl chlorides via photoinduced ligand-to-metal charge transfer
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01488j Sheng-Ping Liu, Lan Yang, Yan-Hong He, Zhi Guan
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01488j Sheng-Ping Liu, Lan Yang, Yan-Hong He, Zhi Guan
|
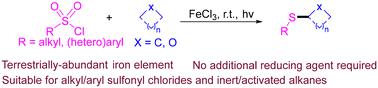
The construction of C(sp3)–S bonds through C(sp3)–H activation holds significant value in the synthesis of sulfides. However, the vast majority of strategies primarily involve the use of electron-rich sulfur sources (such as sulfinates and thioalcohols) to thiolate activated and thermodynamically favored C(sp3)–H bonds. Therefore, further expanding the substrate range of sulfur sources and various C(sp3)–H bonds would provide an entry for the synthesis of unique structural molecules. Here, we report a FeCl3/HCl synergistic catalytic approach that enables the formation of C(sp3)–S bonds via reductive deoxygenation cross-coupling of activated/inert C(sp3)–H and aryl/alkyl sulfonyl chlorides without the need for external reductants. Mechanism studies reveal that HCl plays a crucial role in the thiolation of inert C(sp3)–H bonds, not only enhancing the catalytic activity of FeCl3 but also forming electron donor–acceptor (EDA) complexes with sulfonyl chlorides.
中文翻译:

通过光诱导配体-金属电荷转移将 C(sp3)–H 与磺酰氯铁催化的硫醇化
通过 C(sp3)-H 活化构建 C(sp3)-S 键在硫化物的合成中具有重要价值。然而,绝大多数策略主要涉及使用富电子硫源(如亚磺酸盐和硫醇)来硫代化活化和热力学上有利的 C(sp3)-H 键。因此,进一步扩大硫源和各种 C(sp3)-H 键的底物范围将为合成独特结构分子提供入口。在这里,我们报道了一种 FeCl3/HCl 协同催化方法,该方法能够通过活化/惰性 C(sp3)-H 和芳基/烷基磺酰氯的还原脱氧交叉偶联形成 C(sp3)-S 键,而无需外部还原剂。机理研究表明,HCl 在惰性 C(sp3)-H 键的硫醇化中起着至关重要的作用,不仅增强了 FeCl3 的催化活性,而且还与磺酰氯形成电子供体-受体 (EDA) 复合物。
更新日期:2024-10-29
中文翻译:

通过光诱导配体-金属电荷转移将 C(sp3)–H 与磺酰氯铁催化的硫醇化
通过 C(sp3)-H 活化构建 C(sp3)-S 键在硫化物的合成中具有重要价值。然而,绝大多数策略主要涉及使用富电子硫源(如亚磺酸盐和硫醇)来硫代化活化和热力学上有利的 C(sp3)-H 键。因此,进一步扩大硫源和各种 C(sp3)-H 键的底物范围将为合成独特结构分子提供入口。在这里,我们报道了一种 FeCl3/HCl 协同催化方法,该方法能够通过活化/惰性 C(sp3)-H 和芳基/烷基磺酰氯的还原脱氧交叉偶联形成 C(sp3)-S 键,而无需外部还原剂。机理研究表明,HCl 在惰性 C(sp3)-H 键的硫醇化中起着至关重要的作用,不仅增强了 FeCl3 的催化活性,而且还与磺酰氯形成电子供体-受体 (EDA) 复合物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号