当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic arylation/alkylation of olefins/alkynes via halogen-atom transfer mediated by NHC-BH3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01562b Xinhan Li, Yao Zhong, Fengsong Tan, Yusong Fei, Xiaohan Zhao, Jianbin Xu, Baomin Fan
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01562b Xinhan Li, Yao Zhong, Fengsong Tan, Yusong Fei, Xiaohan Zhao, Jianbin Xu, Baomin Fan
|
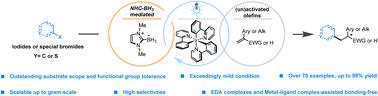
A versatile method for radical reductive cross-coupling of iodides and activated olefins under mild conditions, facilitated by NHC-BH3 through photocatalysis, was developed. This method exhibits high efficiency and regioselectivity, achieving yields of up to 98% for aryl, heterocyclic, and alkyl iodides. The reaction employs a minimal amount (1.0 mol%) of photocatalyst and can be performed on a gram scale. Importantly, this approach avoids the use of transition metal co-catalysts, eliminating the need for traditional C–I bond cleavage steps such as oxidative addition, migration insertion, and halogen exchange. This work unveils a novel mechanism for the reductive coupling of iodides in a photocatalytic NHC-BH3 system, providing insights into iodide modification and the functional exploration of NHC-BH3.
中文翻译:

NHC-BH3 介导的卤素原子转移对烯烃/炔烃进行光催化芳基化/烷基化反应
开发了一种在温和条件下碘化物和活性烯烃自由基还原交叉偶联的通用方法,由 NHC-BH3 通过光催化促进。该方法具有高效率和区域选择性,对芳基、杂环和烷基碘化物的收率高达 98%。该反应使用最少量 (1.0 mol%) 的光催化剂,并且可以在克级上进行。重要的是,这种方法避免了使用过渡金属助催化剂,无需传统的 C-I 键裂解步骤,例如氧化加成、迁移插入和卤素交换。这项工作揭示了光催化 NHC-BH3 系统中碘化物还原偶联的新机制,为碘化物改性和 NHC-BH3 的功能探索提供了见解。
更新日期:2024-11-01
中文翻译:

NHC-BH3 介导的卤素原子转移对烯烃/炔烃进行光催化芳基化/烷基化反应
开发了一种在温和条件下碘化物和活性烯烃自由基还原交叉偶联的通用方法,由 NHC-BH3 通过光催化促进。该方法具有高效率和区域选择性,对芳基、杂环和烷基碘化物的收率高达 98%。该反应使用最少量 (1.0 mol%) 的光催化剂,并且可以在克级上进行。重要的是,这种方法避免了使用过渡金属助催化剂,无需传统的 C-I 键裂解步骤,例如氧化加成、迁移插入和卤素交换。这项工作揭示了光催化 NHC-BH3 系统中碘化物还原偶联的新机制,为碘化物改性和 NHC-BH3 的功能探索提供了见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号