当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of bambusurils with perfluoroalkylthiobenzyl groups as highly potent halide receptors
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01746c Matúš Chvojka, Hennie Valkenier, Vladimír Šindelář
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01746c Matúš Chvojka, Hennie Valkenier, Vladimír Šindelář
|
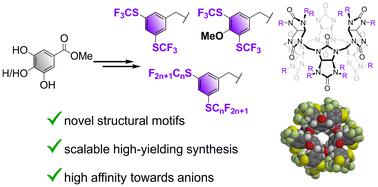
The preparation of anion receptors with ultrahigh binding affinities is an important, yet challenging, topic of supramolecular chemistry. The search for new structural motifs which would enhance the performance of anion receptors is therefore an important task. In this context, we report the synthesis of novel fluorinated bambus[6]urils that incorporate unique benzyl substituents with perfluoroalkylthio groups, aimed at enhancing their anion receptor capabilities. The synthetic strategy developed allows for the efficient preparation of these structural motifs. Ultrahigh stability of the complexes between halides and the prepared bambus[6]urils was observed and quantified using 19F NMR competition experiments. Replacing –CF3 groups on benzylated bambus[6]urils by –SCF3 groups increased the affinity of the macrocycles towards anions and provided the strongest iodide receptor reported with a binding affinity of 4 × 1013 M−1 in acetonitrile.
中文翻译:

合成具有全氟烷基硫代苄基作为高效卤化物受体的 bambusuril
制备具有超高结合亲和力的阴离子受体是超分子化学中一个重要但具有挑战性的课题。因此,寻找能够增强阴离子受体性能的新结构基序是一项重要任务。在此背景下,我们报道了新型氟化 bambus[6]urils 的合成,这些脲基结合了独特的苄基取代基和全氟烷基硫代基团,旨在增强其阴离子受体能力。开发的合成策略允许有效地制备这些结构基序。使用 19F NMR 竞争实验观察卤化物和制备的 bambus[6]urils 之间的复合物的超高稳定性并进行定量。用 –SCF3 基团替换苄基化 bambus[6] 尿基上的 –CF3 基团增加了大环对阴离子的亲和力,并提供了最强的碘受体,据报道在乙腈中的结合亲和力为 4 × 1013 M-1。
更新日期:2024-11-01
中文翻译:

合成具有全氟烷基硫代苄基作为高效卤化物受体的 bambusuril
制备具有超高结合亲和力的阴离子受体是超分子化学中一个重要但具有挑战性的课题。因此,寻找能够增强阴离子受体性能的新结构基序是一项重要任务。在此背景下,我们报道了新型氟化 bambus[6]urils 的合成,这些脲基结合了独特的苄基取代基和全氟烷基硫代基团,旨在增强其阴离子受体能力。开发的合成策略允许有效地制备这些结构基序。使用 19F NMR 竞争实验观察卤化物和制备的 bambus[6]urils 之间的复合物的超高稳定性并进行定量。用 –SCF3 基团替换苄基化 bambus[6] 尿基上的 –CF3 基团增加了大环对阴离子的亲和力,并提供了最强的碘受体,据报道在乙腈中的结合亲和力为 4 × 1013 M-1。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号