Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detailed mechanistic studies on PNN-palladium pincer complex catalyzed Suzuki-Miyaura cross-coupling reaction proceeding through a PdII/PdIII/PdIV catalytic cycle
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.jcat.2024.115825 Gazal Sabharwal, Khilesh C. Dwivedi, Chandan Das, Thakur Rochak Kumar Rana, Arnab Dutta, Gopalan Rajaraman, Maravanji S. Balakrishna
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.jcat.2024.115825 Gazal Sabharwal, Khilesh C. Dwivedi, Chandan Das, Thakur Rochak Kumar Rana, Arnab Dutta, Gopalan Rajaraman, Maravanji S. Balakrishna
|
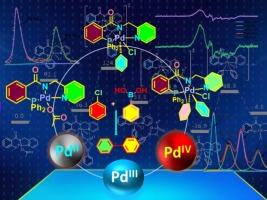
In this paper, synthesis, Pd and Pt complexes and catalytic investigations of a tridentate pincer ligand 2-(diphenylphosphaneyl)-N-(pyridine-2-ylmethyl)benzamide, {(o -PPh2 )C6 H4 C(O)N(H)CH2 (C6 H5 N)} (1 ), (hereafter referred to as “PN(H)N” and its anionic form as “PNN”) is described. Reaction of 1 with MCl2 (COD) resulted in pincer complexes [MCl(PNN-κ3 -P,N,N)] (Pd 2 , Pt 3 ), whereas the same reaction with Pd(OAc)2 afforded [CH3 C(O)OPd(PNN-κ3 -P,N,N)] (4 ). The structures of all of the compounds are confirmed by single-crystal X-ray analysis. The palladium complex 4 promoted the Suzuki-Miyaura cross-coupling reaction between aryl halides and boronic acids to form the corresponding biphenyls in excellent yields. The catalytic investigation supported by spectroscopic studies and DFT calculations suggested the involvement of PdII /PdIV catalytic cycle via a short lived PdIII intermediate, which transforms into PdIV species rapidly. The catalytic process proceeded with a very low catalyst loading under relatively mild reaction conditions.
中文翻译:

PNN-钯钳络合物催化 Suzuki-Miyaura 交叉偶联反应通过 PdII/PdIII/PdIV 催化循环的详细机理研究
本文介绍了三齿钳形配体2-(二苯基膦酰基)-N-(吡啶-2-基甲基)苯甲酰胺,{(o-PPh2)C6H4C(O)N(H)CH2(C6H5N)}(1),(以下简称“PN(H)N”,其阴离子形式为“PNN”)的合成、Pd和Pt配合物以及催化研究。1 与 MCl2 (COD) 反应产生钳形复合物 [MCl(PNN-κ3-P,N,N)] (Pd 2, Pt 3),而与 Pd(OAc)2 的相同反应得到 [CH3C(O)OPd(PNN-κ3-P,N,N)] (4)。所有化合物的结构都通过单晶 X 射线分析得到证实。钯配合物 4 促进了芳基卤化物和硼酸之间的 Suzuki-Miyaura 交叉偶联反应,以优异的产率形成相应的联苯。光谱研究和 DFT 计算支持的催化研究表明,PdII/PdIV 催化循环通过短寿命的 PdIII 中间体参与,该中间体迅速转化为 PdIV 物质。催化过程在相对温和的反应条件下以非常低的催化剂负载进行。
更新日期:2024-10-28
中文翻译:

PNN-钯钳络合物催化 Suzuki-Miyaura 交叉偶联反应通过 PdII/PdIII/PdIV 催化循环的详细机理研究
本文介绍了三齿钳形配体2-(二苯基膦酰基)-N-(吡啶-2-基甲基)苯甲酰胺,{(o-PPh2)C6H4C(O)N(H)CH2(C6H5N)}(1),(以下简称“PN(H)N”,其阴离子形式为“PNN”)的合成、Pd和Pt配合物以及催化研究。1 与 MCl2 (COD) 反应产生钳形复合物 [MCl(PNN-κ3-P,N,N)] (Pd 2, Pt 3),而与 Pd(OAc)2 的相同反应得到 [CH3C(O)OPd(PNN-κ3-P,N,N)] (4)。所有化合物的结构都通过单晶 X 射线分析得到证实。钯配合物 4 促进了芳基卤化物和硼酸之间的 Suzuki-Miyaura 交叉偶联反应,以优异的产率形成相应的联苯。光谱研究和 DFT 计算支持的催化研究表明,PdII/PdIV 催化循环通过短寿命的 PdIII 中间体参与,该中间体迅速转化为 PdIV 物质。催化过程在相对温和的反应条件下以非常低的催化剂负载进行。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号