当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small-molecule targeting BCAT1-mediated BCAA metabolism inhibits the activation of SHOC2-RAS-ERK to induce apoptosis of Triple-negative breast cancer cells
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.jare.2024.10.021 Ling Huang, Guanjun Li, Ying Zhang, Ruishen Zhuge, Shijie Qin, Jinjun Qian, Ruixing Chen, Yin Kwan Wong, Huan Tang, Peili Wang, Wei Xiao, Jigang Wang
中文翻译:

靶向 BCAT1 介导的 BCAA 代谢的小分子抑制 SHOC2-RAS-ERK 的激活以诱导三阴性乳腺癌细胞凋亡
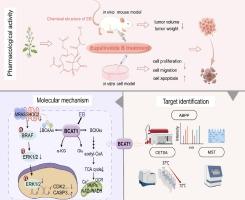
三阴性乳腺癌 (TNBC) 是乳腺癌中最恶性、预后最差的亚型。探索 TNBC 的新型致癌因素和治疗药物仍然是改善预后的重点。支链氨基酸转氨酶 1 (BCAT1) 是支链氨基酸 (BCAA) 代谢中的关键酶,与各种肿瘤发展有关,但其在 TNBC 中的致癌功能和机制仍不清楚。Eupalinolide B (EB) 是一种天然衍生的具有抗肿瘤活性的小分子,但其在 TNBC 中的作用仍然未知。
通过探讨 EB 抑制 TNBC 的靶点和药理机制,本研究旨在发现 TNBC 的新治疗靶点和潜在抑制剂,并阐明 TNBC 的新致病机制。
使用小鼠模型和细胞表型实验研究 EB 对 TNBC 的抑制作用。利用基于活性的蛋白质分析 (ABPP) 技术、下拉 WB、CETSA-WB 和 MST 来发现和验证 EB 的靶点。通过临床数据分析和生化实验确定 BCAT1 的致癌作用。为了阐明 EB 抑制 TNBC 的机制,使用了许多方法,包括但不限于 HPLC 和蛋白质组学测序。
我们发现 EB 显着抑制 TNBC 进展。我们确定 BCAT1 是 EB 的直接靶标,并证实 BCAT1 对 TNBC 的发展至关重要。EB 抑制 BCAT1 参与的 BCAA 代谢,减少 BCAA (包括 Leu、Ile 和 Val) 的合成,从而抑制 SHOC2 (一种富含 Leu 的重复蛋白) 表达和下游 SHOC2 参与的 RAS-ERK 信号通路,最终导致 TNBC 细胞凋亡。
总的来说,这项研究不仅阐明了 BCAT1 及其下游 SHOC2-RAS-ERK 信号轴在 TNBC 进展中的致癌作用,而且还为靶向 BCAT1 或 BCAA 代谢的潜在疗法开辟了途径(单独使用 EB 或与其抑制剂坎地沙坦联合使用)治疗 TNBC。
更新日期:2024-10-28
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.jare.2024.10.021 Ling Huang, Guanjun Li, Ying Zhang, Ruishen Zhuge, Shijie Qin, Jinjun Qian, Ruixing Chen, Yin Kwan Wong, Huan Tang, Peili Wang, Wei Xiao, Jigang Wang
|
Introduction
Triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancer with the worst prognosis. Exploring novel carcinogenic factors and therapeutic drugs for TNBC remains a focus to improve prognosis. Branched-chain amino acid transaminase 1 (BCAT1), a crucial enzyme in branched-chain amino acid (BCAA) metabolism, has been linked to various tumor developments, but its carcinogenic function and mechanism in TNBC remain unclear. Eupalinolide B (EB) is a naturally-derived small-molecule with anti-tumor activity, but its role in TNBC remains unknown.Objectives
By exploring the targets and pharmacological mechanisms of EB in inhibiting TNBC, this study aimed to discover novel therapeutic targets and potential inhibitors for TNBC, and elucidate novel pathogenic mechanisms of TNBC.Methods
The inhibitory effect of EB on TNBC was investigated using mouse models and cellular phenotypic experiments. Activity-based protein profiling (ABPP) technology, pull down-WB, CETSA-WB and MST were utilized to discover and validate the targets of EB. The oncogenic role of BCAT1 was determined through clinical data analysis and biochemical experiments. To elucidate the mechanism by which EB inhibited TNBC, many methods, including but not limited to HPLC and proteomic sequencing were used.Results
We found that EB significantly inhibited TNBC progression. We identified BCAT1 as the direct target of EB and confirmed that BCAT1 was critical for TNBC development. EB inhibited BCAT1-involved BCAA metabolism to reduce the synthesis of BCAAs (including Leu, Ile, and Val), thereby inhibiting SHOC2 (a Leu-rich repeat protein) expression and the downstream SHOC2-participating RAS-ERK signaling pathway, ultimately leading to apoptosis of TNBC cells.Conclusion
Collectively, this study not only elucidates the oncogenic role of BCAT1 and its downstream SHOC2-RAS-ERK signaling axis in TNBC progression but also opens up avenues for potential therapies targeting BCAT1 or BCAA metabolism (using EB alone or in combination with its inhibitor candesartan) for TNBC treatment.中文翻译:

靶向 BCAT1 介导的 BCAA 代谢的小分子抑制 SHOC2-RAS-ERK 的激活以诱导三阴性乳腺癌细胞凋亡
介绍
三阴性乳腺癌 (TNBC) 是乳腺癌中最恶性、预后最差的亚型。探索 TNBC 的新型致癌因素和治疗药物仍然是改善预后的重点。支链氨基酸转氨酶 1 (BCAT1) 是支链氨基酸 (BCAA) 代谢中的关键酶,与各种肿瘤发展有关,但其在 TNBC 中的致癌功能和机制仍不清楚。Eupalinolide B (EB) 是一种天然衍生的具有抗肿瘤活性的小分子,但其在 TNBC 中的作用仍然未知。
目标
通过探讨 EB 抑制 TNBC 的靶点和药理机制,本研究旨在发现 TNBC 的新治疗靶点和潜在抑制剂,并阐明 TNBC 的新致病机制。
方法
使用小鼠模型和细胞表型实验研究 EB 对 TNBC 的抑制作用。利用基于活性的蛋白质分析 (ABPP) 技术、下拉 WB、CETSA-WB 和 MST 来发现和验证 EB 的靶点。通过临床数据分析和生化实验确定 BCAT1 的致癌作用。为了阐明 EB 抑制 TNBC 的机制,使用了许多方法,包括但不限于 HPLC 和蛋白质组学测序。
结果
我们发现 EB 显着抑制 TNBC 进展。我们确定 BCAT1 是 EB 的直接靶标,并证实 BCAT1 对 TNBC 的发展至关重要。EB 抑制 BCAT1 参与的 BCAA 代谢,减少 BCAA (包括 Leu、Ile 和 Val) 的合成,从而抑制 SHOC2 (一种富含 Leu 的重复蛋白) 表达和下游 SHOC2 参与的 RAS-ERK 信号通路,最终导致 TNBC 细胞凋亡。
结论
总的来说,这项研究不仅阐明了 BCAT1 及其下游 SHOC2-RAS-ERK 信号轴在 TNBC 进展中的致癌作用,而且还为靶向 BCAT1 或 BCAA 代谢的潜在疗法开辟了途径(单独使用 EB 或与其抑制剂坎地沙坦联合使用)治疗 TNBC。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号