Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic analysis of the self-discharge of the NiOOH OER active phase in KOH electrolyte: insights from in-situ Raman and UV–Vis reflectance spectroscopies
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-26 , DOI: 10.1016/j.jcat.2024.115823 Harol Moreno Fernández, Achim Alkemper, Kai Wang, Crizaldo Jr. Mempin, Julia Gallenbeger, Jan P. Hofmann
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-26 , DOI: 10.1016/j.jcat.2024.115823 Harol Moreno Fernández, Achim Alkemper, Kai Wang, Crizaldo Jr. Mempin, Julia Gallenbeger, Jan P. Hofmann
|
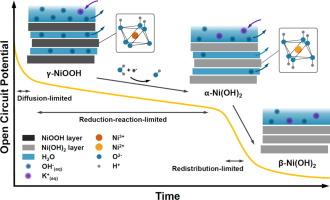
NiOOH has been established as the active phase of NiO-based electrocatalysts in the alkaline Oxygen Evolution Reaction (OER). Here, we investigate the self-discharge behavior of NiOOH electrodes under open circuit potential (OCP) conditions in 1 M KOH electrolyte by monitoring phase changes via in-situ Raman and UV–Vis reflectance spectroscopies and performing kinetic analyses on the OCP and spectroscopic data. Our findings reveal a linear phase change from NiOOH to Ni(OH)2 over time, indicative of a 0th -order reduction reaction. Contrarily, the OCP evolution associated with this phase reduction displayed a combination of linear and exponential decay patterns as a result of various kinetics, including Faradaic processes and diffusion-controlled mechanisms, influencing the self-discharge potential over 1.25 V (vs RHE). An additional linear region at lower potentials (<1.25 V (vs RHE)) suggests that charge redistribution due to the phase change from α-Ni(OH)2 to β-Ni(OH)2 dominates the self-discharge, a behavior confirmed by in-situ UV–Vis reflectance spectroscopy. These findings highlight the effectiveness of combining in-situ Raman and UV–Vis spectroscopy with electrochemical data for real-time monitoring of electrochemical processes, here potential-dependent electrocatalyst phase changes, leading to a more detailed and accurate understanding of the dynamic behavior, phase change kinetics, and self-discharge behaviors of solid electrocatalysts that can guide the design of more efficient and durable energy storage and conversion materials.
中文翻译:

KOH 电解液中 NiOOH OER 活性相自放电的动力学分析:来自原位拉曼光谱和紫外-可见光反射光谱的见解
NiOOH 已被确定为碱性析氧反应 (OER) 中 NiO 基电催化剂的活性相。在这里,我们通过原位拉曼和紫外-可见反射光谱监测相变,并对 OCP 和光谱数据进行动力学分析,研究了 NiOOH 电极在 1 M KOH 电解质中开路电位 (OCP) 条件下的自放电行为。我们的研究结果揭示了随着时间的推移从 NiOOH 到 Ni(OH)2 的线性相变,表明 0 级还原反应。相反,与这种相简化相关的 OCP 演变显示出线性和指数衰减模式的组合,这是各种动力学的结果,包括法拉第过程和扩散控制机制,影响了超过 1.25 V (vs RHE) 的自放电电位。较低电位下的另一个线性区域 (<1.25 V (vs RHE)) 表明,由于 α-Ni(OH)2 到 β-Ni(OH)2 的相变导致的电荷重新分布主导了自放电,原位紫外-可见光反射光谱证实了这一行为。这些发现强调了将原位拉曼光谱和紫外-可见光谱与电化学数据相结合以实时监测电化学过程的有效性,其中电势依赖性电催化剂的相变,从而更详细、更准确地了解固体电催化剂的动态行为、相变动力学和自放电行为,从而指导更高效、更耐用的储能和转换材料的设计。
更新日期:2024-10-26
中文翻译:

KOH 电解液中 NiOOH OER 活性相自放电的动力学分析:来自原位拉曼光谱和紫外-可见光反射光谱的见解
NiOOH 已被确定为碱性析氧反应 (OER) 中 NiO 基电催化剂的活性相。在这里,我们通过原位拉曼和紫外-可见反射光谱监测相变,并对 OCP 和光谱数据进行动力学分析,研究了 NiOOH 电极在 1 M KOH 电解质中开路电位 (OCP) 条件下的自放电行为。我们的研究结果揭示了随着时间的推移从 NiOOH 到 Ni(OH)2 的线性相变,表明 0 级还原反应。相反,与这种相简化相关的 OCP 演变显示出线性和指数衰减模式的组合,这是各种动力学的结果,包括法拉第过程和扩散控制机制,影响了超过 1.25 V (vs RHE) 的自放电电位。较低电位下的另一个线性区域 (<1.25 V (vs RHE)) 表明,由于 α-Ni(OH)2 到 β-Ni(OH)2 的相变导致的电荷重新分布主导了自放电,原位紫外-可见光反射光谱证实了这一行为。这些发现强调了将原位拉曼光谱和紫外-可见光谱与电化学数据相结合以实时监测电化学过程的有效性,其中电势依赖性电催化剂的相变,从而更详细、更准确地了解固体电催化剂的动态行为、相变动力学和自放电行为,从而指导更高效、更耐用的储能和转换材料的设计。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号