当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Phosphorylation on the Physiological Form of Human alpha-Synuclein in Aqueous Solution
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-10-27 , DOI: 10.1021/acs.jcim.4c01172 Emile de Bruyn, Anton Emil Dorn, Giulia Rossetti, Claudio Fernandez, Tiago F. Outeiro, Jörg B. Schulz, Paolo Carloni
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-10-27 , DOI: 10.1021/acs.jcim.4c01172 Emile de Bruyn, Anton Emil Dorn, Giulia Rossetti, Claudio Fernandez, Tiago F. Outeiro, Jörg B. Schulz, Paolo Carloni
|
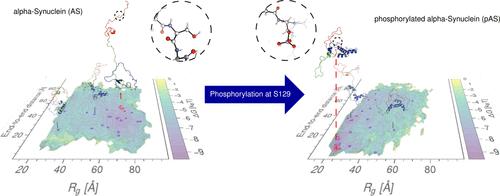
Serine 129 can be phosphorylated in pathological inclusions formed by the intrinsically disordered protein human α-synuclein (AS), a key player in Parkinson’s disease and other synucleinopathies. Here, molecular simulations provide insight into the structural ensemble of phosphorylated AS. The simulations allow us to suggest that phosphorylation significantly impacts the structural content of the physiological AS conformational ensemble in aqueous solution, as the phosphate group is mostly solvated. The hydrophobic region of AS contains β-hairpin structures, which may increase the propensity of the protein to undergo amyloid formation, as seen in the nonphysiological (nonacetylated) form of the protein in a recent molecular simulation study. Our findings are consistent with existing experimental data with the caveat of the observed limitations of the force field for the phosphorylated moiety.
中文翻译:

磷酸化对水溶液中人 α-突触核蛋白生理形式的影响
丝氨酸 129 可在固有无序蛋白人 α-突触核蛋白 (AS) 形成的病理包涵体中被磷酸化,AS是帕金森病和其他突触核蛋白病的关键参与者。在这里,分子模拟提供了对磷酸化 AS 结构集合的见解。模拟使我们能够表明,磷酸化会显著影响水溶液中生理 AS 构象集合的结构含量,因为磷酸基团大部分是溶剂化的。AS 的疏水区域包含β发夹结构,这可能会增加蛋白质发生淀粉样蛋白形成的倾向,如最近的分子模拟研究中蛋白质的非生理性(非乙酰化)形式所见。我们的研究结果与现有的实验数据一致,但需要注意的是观察到的磷酸化部分的力场的局限性。
更新日期:2024-10-28
中文翻译:

磷酸化对水溶液中人 α-突触核蛋白生理形式的影响
丝氨酸 129 可在固有无序蛋白人 α-突触核蛋白 (AS) 形成的病理包涵体中被磷酸化,AS是帕金森病和其他突触核蛋白病的关键参与者。在这里,分子模拟提供了对磷酸化 AS 结构集合的见解。模拟使我们能够表明,磷酸化会显著影响水溶液中生理 AS 构象集合的结构含量,因为磷酸基团大部分是溶剂化的。AS 的疏水区域包含β发夹结构,这可能会增加蛋白质发生淀粉样蛋白形成的倾向,如最近的分子模拟研究中蛋白质的非生理性(非乙酰化)形式所见。我们的研究结果与现有的实验数据一致,但需要注意的是观察到的磷酸化部分的力场的局限性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号