Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insight into rabies virus neutralization revealed by an engineered antibody scaffold
Structure ( IF 4.4 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.str.2024.10.002 Ashwini Kedari, Rommel Iheozor-Ejiofor, Petja Salminen, Hasan Uğurlu, Anna R. Mäkelä, Lev Levanov, Olli Vapalahti, Vesa P. Hytönen, Kalle Saksela, Ilona Rissanen
Structure ( IF 4.4 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.str.2024.10.002 Ashwini Kedari, Rommel Iheozor-Ejiofor, Petja Salminen, Hasan Uğurlu, Anna R. Mäkelä, Lev Levanov, Olli Vapalahti, Vesa P. Hytönen, Kalle Saksela, Ilona Rissanen
|
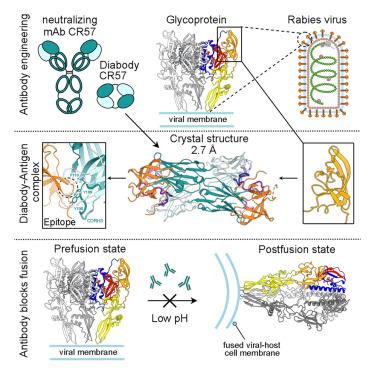
Host-cell entry of the highly pathogenic rabies virus (RABV) is mediated by glycoprotein (G) spikes, which also comprise the primary target for the humoral immune response. RABV glycoprotein (RABV-G) displays several antigenic sites that are targeted by neutralizing monoclonal antibodies (mAbs). In this study, we determined the epitope of a potently neutralizing human mAb, CR57, which we engineered into a diabody format to facilitate crystallization. We report the crystal structure of the CR57 diabody alone at 2.38 Å resolution, and in complex with RABV-G domain III at 2.70 Å resolution. The CR57−RABV-G structure reveals critical interactions at the antigen interface, which target the conserved “KLCGVL” peptide and residues proximal to it on RABV-G. Structural analysis combined with a cell-cell fusion assay demonstrates that CR57 effectively inhibits RABV-G-mediated fusion by obstructing the fusogenic transitions of the spike protein. Altogether, this investigation provides a structural perspective on RABV inhibition by a potently neutralizing human antibody.
中文翻译:

通过工程抗体支架揭示狂犬病病毒中和的结构见解
高致病性狂犬病病毒 (RABV) 的宿主细胞进入是由糖蛋白 (G) 刺突介导的,糖蛋白 (G) 刺突也构成了体液免疫反应的主要靶标。RABV 糖蛋白 (RABV-G) 显示几个抗原位点,这些位点被中和单克隆抗体 (mAb) 靶向。在这项研究中,我们确定了一种有效中和人 mAb CR57 的表位,并将其设计成 diabody 形式以促进结晶。我们以 2.38 Å 的分辨率单独报道了 CR57 双体的晶体结构,并以 2.70 Å 的分辨率报道了与 RABV-G 结构域 III 的复合物的晶体结构。CR57−RABV-G 结构揭示了抗原界面的关键相互作用,这些相互作用靶向 RABV-G 上保守的“KLCGVL”肽及其近端的残基。结构分析结合细胞间融合测定表明,CR57 通过阻断刺突蛋白的融合转变,有效抑制 RABV-G 介导的融合。总而言之,这项研究为有效中和人类抗体抑制 RABV 提供了结构视角。
更新日期:2024-10-28
中文翻译:

通过工程抗体支架揭示狂犬病病毒中和的结构见解
高致病性狂犬病病毒 (RABV) 的宿主细胞进入是由糖蛋白 (G) 刺突介导的,糖蛋白 (G) 刺突也构成了体液免疫反应的主要靶标。RABV 糖蛋白 (RABV-G) 显示几个抗原位点,这些位点被中和单克隆抗体 (mAb) 靶向。在这项研究中,我们确定了一种有效中和人 mAb CR57 的表位,并将其设计成 diabody 形式以促进结晶。我们以 2.38 Å 的分辨率单独报道了 CR57 双体的晶体结构,并以 2.70 Å 的分辨率报道了与 RABV-G 结构域 III 的复合物的晶体结构。CR57−RABV-G 结构揭示了抗原界面的关键相互作用,这些相互作用靶向 RABV-G 上保守的“KLCGVL”肽及其近端的残基。结构分析结合细胞间融合测定表明,CR57 通过阻断刺突蛋白的融合转变,有效抑制 RABV-G 介导的融合。总而言之,这项研究为有效中和人类抗体抑制 RABV 提供了结构视角。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号