当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A hierarchical hepatic de novo lipogenesis substrate supply network utilizing pyruvate, acetate, and ketones
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.cmet.2024.10.013 Adam J. Rauckhorst, Ryan D. Sheldon, Daniel J. Pape, Adnan Ahmed, Kelly C. Falls-Hubert, Ronald A. Merrill, Reid F. Brown, Kshitij Deshmukh, Thomas A. Vallim, Stanislaw Deja, Shawn C. Burgess, Eric B. Taylor
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.cmet.2024.10.013 Adam J. Rauckhorst, Ryan D. Sheldon, Daniel J. Pape, Adnan Ahmed, Kelly C. Falls-Hubert, Ronald A. Merrill, Reid F. Brown, Kshitij Deshmukh, Thomas A. Vallim, Stanislaw Deja, Shawn C. Burgess, Eric B. Taylor
|
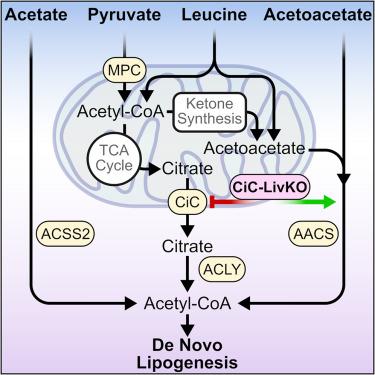
Hepatic de novo lipogenesis (DNL) is a fundamental physiologic process that is often pathogenically elevated in metabolic disease. Treatment is limited by incomplete understanding of the metabolic pathways supplying cytosolic acetyl-CoA, the obligate precursor to DNL, including their interactions and proportional contributions. Here, we combined extensive 13C tracing with liver-specific knockout of key mitochondrial and cytosolic proteins mediating cytosolic acetyl-CoA production. We show that the mitochondrial pyruvate carrier (MPC) and ATP-citrate lyase (ACLY) gate the major hepatic lipogenic acetyl-CoA production pathway, operating in parallel with acetyl-CoA synthetase 2 (ACSS2). Given persistent DNL after mitochondrial citrate carrier (CiC) and ACSS2 double knockout, we tested the contribution of exogenous and leucine-derived acetoacetate to acetoacetyl-CoA synthetase (AACS)-dependent DNL. CiC knockout increased acetoacetate-supplied hepatic acetyl-CoA production and DNL, indicating that ketones function as mitochondrial-citrate reciprocal DNL precursors. By delineating a mitochondrial-cytosolic DNL substrate supply network, these findings may inform strategies to therapeutically modulate DNL.
中文翻译:

利用丙酮酸、乙酸盐和酮体的分层肝脏从头脂肪生成底物供应网络
肝脏新发脂肪生成 (DNL) 是一个基本的生理过程,在代谢性疾病中通常呈致病性升高。由于对提供胞质乙酰辅酶 A(DNL 的专性前体)的代谢途径不完全了解,包括它们的相互作用和比例贡献,治疗受到限制。在这里,我们将广泛的 13C 示踪与介导胞质乙酰辅酶 A 产生的关键线粒体和胞质蛋白的肝脏特异性敲除相结合。我们表明,线粒体丙酮酸载体 (MPC) 和 ATP-柠檬酸裂解酶 (ACLY) 是肝脏脂肪生成乙酰辅酶 A 产生的主要途径,与乙酰辅酶 A 合成酶 2 (ACSS2) 平行运作。鉴于线粒体柠檬酸盐载体 (CiC) 和 ACSS2 双敲除后的持续 DNL,我们测试了外源性和亮氨酸衍生的乙酰乙酸酯对乙酰乙酰辅酶 A 合成酶 (AACS) 依赖性 DNL 的贡献。CiC 敲除增加了乙酰乙酸提供的肝脏乙酰辅酶 A 产生和 DNL,表明酮体作为线粒体-柠檬酸盐互惠 DNL 前体发挥作用。通过描绘线粒体-胞质溶质 DNL 底物供应网络,这些发现可能为治疗调节 DNL 的策略提供信息。
更新日期:2024-10-28
中文翻译:

利用丙酮酸、乙酸盐和酮体的分层肝脏从头脂肪生成底物供应网络
肝脏新发脂肪生成 (DNL) 是一个基本的生理过程,在代谢性疾病中通常呈致病性升高。由于对提供胞质乙酰辅酶 A(DNL 的专性前体)的代谢途径不完全了解,包括它们的相互作用和比例贡献,治疗受到限制。在这里,我们将广泛的 13C 示踪与介导胞质乙酰辅酶 A 产生的关键线粒体和胞质蛋白的肝脏特异性敲除相结合。我们表明,线粒体丙酮酸载体 (MPC) 和 ATP-柠檬酸裂解酶 (ACLY) 是肝脏脂肪生成乙酰辅酶 A 产生的主要途径,与乙酰辅酶 A 合成酶 2 (ACSS2) 平行运作。鉴于线粒体柠檬酸盐载体 (CiC) 和 ACSS2 双敲除后的持续 DNL,我们测试了外源性和亮氨酸衍生的乙酰乙酸酯对乙酰乙酰辅酶 A 合成酶 (AACS) 依赖性 DNL 的贡献。CiC 敲除增加了乙酰乙酸提供的肝脏乙酰辅酶 A 产生和 DNL,表明酮体作为线粒体-柠檬酸盐互惠 DNL 前体发挥作用。通过描绘线粒体-胞质溶质 DNL 底物供应网络,这些发现可能为治疗调节 DNL 的策略提供信息。






























 京公网安备 11010802027423号
京公网安备 11010802027423号