Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiparameter imaging reveals clinically relevant cancer cell-stroma interaction dynamics in head and neck cancer
Cell ( IF 45.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.cell.2024.09.046 Karolina Punovuori, Fabien Bertillot, Yekaterina A. Miroshnikova, Mirjam I. Binner, Satu-Marja Myllymäki, Gautier Follain, Kai Kruse, Johannes Routila, Teemu Huusko, Teijo Pellinen, Jaana Hagström, Noemi Kedei, Sami Ventelä, Antti Mäkitie, Johanna Ivaska, Sara A. Wickström
Cell ( IF 45.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.cell.2024.09.046 Karolina Punovuori, Fabien Bertillot, Yekaterina A. Miroshnikova, Mirjam I. Binner, Satu-Marja Myllymäki, Gautier Follain, Kai Kruse, Johannes Routila, Teemu Huusko, Teijo Pellinen, Jaana Hagström, Noemi Kedei, Sami Ventelä, Antti Mäkitie, Johanna Ivaska, Sara A. Wickström
|
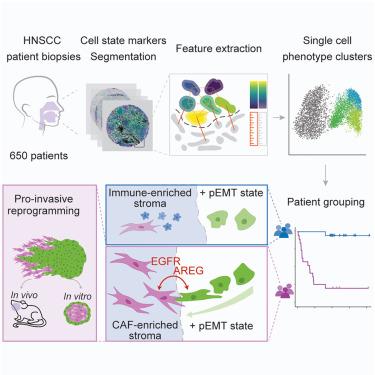
Epithelial tumors are characterized by abundant inter- and intra-tumor heterogeneity, which complicates diagnostics and treatment. The contribution of cancer-stroma interactions to this heterogeneity is poorly understood. Here, we report a paradigm to quantify phenotypic diversity in head and neck squamous cell carcinoma (HNSCC) with single-cell resolution. By combining cell-state markers with morphological features, we identify phenotypic signatures that correlate with clinical features, including metastasis and recurrence. Integration of tumor and stromal signatures reveals that partial epithelial-mesenchymal transition (pEMT) renders disease outcome highly sensitive to stromal composition, generating a strong prognostic and predictive signature. Spatial transcriptomics and subsequent analyses of cancer spheroid dynamics identify the cancer-associated fibroblast-pEMT axis as a nexus for intercompartmental signaling that reprograms pEMT cells into an invasive phenotype. Taken together, we establish a paradigm to identify clinically relevant tumor phenotypes and discover a cell-state-dependent interplay between stromal and epithelial compartments that drives cancer aggression.
中文翻译:

多参数成像揭示了头颈癌中临床相关的癌细胞-基质相互作用动力学
上皮肿瘤的特点是肿瘤间和肿瘤内异质性丰富,这使得诊断和治疗复杂化。癌症-基质相互作用对这种异质性的贡献知之甚少。在这里,我们报告了一种以单细胞分辨率量化头颈部鳞状细胞癌 (HNSCC) 表型多样性的范例。通过将细胞状态标志物与形态学特征相结合,我们确定了与临床特征相关的表型特征,包括转移和复发。肿瘤和基质特征的整合表明,部分上皮-间充质转化 (pEMT) 使疾病结果对基质成分高度敏感,从而产生强烈的预后和预测特征。空间转录组学和随后的癌症球动力学分析将癌症相关的成纤维细胞-pEMT 轴确定为将 pEMT 细胞重编程为侵袭性表型的房室间信号转导的纽带。综上所述,我们建立了一个范式来识别临床相关的肿瘤表型,并发现基质和上皮区室之间驱动癌症侵袭的细胞状态依赖性相互作用。
更新日期:2024-10-28
中文翻译:

多参数成像揭示了头颈癌中临床相关的癌细胞-基质相互作用动力学
上皮肿瘤的特点是肿瘤间和肿瘤内异质性丰富,这使得诊断和治疗复杂化。癌症-基质相互作用对这种异质性的贡献知之甚少。在这里,我们报告了一种以单细胞分辨率量化头颈部鳞状细胞癌 (HNSCC) 表型多样性的范例。通过将细胞状态标志物与形态学特征相结合,我们确定了与临床特征相关的表型特征,包括转移和复发。肿瘤和基质特征的整合表明,部分上皮-间充质转化 (pEMT) 使疾病结果对基质成分高度敏感,从而产生强烈的预后和预测特征。空间转录组学和随后的癌症球动力学分析将癌症相关的成纤维细胞-pEMT 轴确定为将 pEMT 细胞重编程为侵袭性表型的房室间信号转导的纽带。综上所述,我们建立了一个范式来识别临床相关的肿瘤表型,并发现基质和上皮区室之间驱动癌症侵袭的细胞状态依赖性相互作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号