当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogenated solvation structure and preferred Zn (002) deposition via trace additive towards high reversibility for aqueous zinc-ion batteries
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.ensm.2024.103869 Xue Chen, Shijia Li, Kai Wang, Huiling Zhao, Guanjie He, Ying Bai
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.ensm.2024.103869 Xue Chen, Shijia Li, Kai Wang, Huiling Zhao, Guanjie He, Ying Bai
|
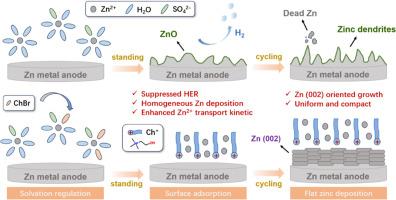
Tremendous progress has been achieved in cathode materials for aqueous zinc-ion batteries (AZIBs), however their practical applications are hindered by the poor cycling stability of Zn anode. Herein, amphiphilic choline bromine (ChBr) is introduced as additive into highly-concentrated ZnSO4 aqueous electrolyte, which not only regulates the traditional Zn2+ solvation structure but also establishes an electrostatic shielding layer at electrolyte-anode interface. Compared to the pristine 3 M ZnSO4 electrolyte, ChBr-modified ZnSO4 electrolyte (ZSO-ChBr) is proven effective in promoting the reversibility of zinc plating/stripping and the preferred growth of Zn (002) plane, as well as suppressing the hydrogen evolution and side reactions on Zn anode surface. As a result, Zn||Zn symmetric cell in optimal ZSO-ChBr electrolyte could harvest a remarkable lifespan over 6000 h at 5 mA cm−2 and 1 mAh cm−2 , besides a highly-reversible zinc plating/stripping process over 1500 cycles was achieved in Zn||Cu asymmetric cell. Moreover, the Zn||MnO2 full cell could exhibit an excellent rate capability and the cycling stability with a capacity retention of 80 % after 400 cycles. This work provides an integrated strategy of electrolyte engineering to elevate the desolvation kinetics of Zn2+ at anode-electrolyte interface and enhance the cycling stability of Zn anode, effectively improving the electrochemical performances of aqueous zinc-ion batteries (AZIBs) and promoting the development of various energy storage systems.
中文翻译:

卤化溶剂化结构和优选的 Zn (002) 通过痕量添加剂沉积,以实现水性锌离子电池的高可逆性
水系锌离子电池 (AZIB) 的正极材料已经取得了巨大的进展,但其实际应用受到 Zn 负极循环稳定性差的阻碍。本文将两亲性胆碱溴 (ChBr) 作为添加剂引入高浓度 ZnSO4 水性电解质中,不仅调节了传统的 Zn2+ 溶剂化结构,而且在电解质-阳极界面建立了静电屏蔽层。与原始的 3 M ZnSO4 电解质相比,ChBr 改性的 ZnSO4 电解质 (ZSO-ChBr) 被证明可有效促进镀锌/剥离的可逆性和 Zn (002) 平面的优先生长,以及抑制 Zn 负极表面的析氢和副反应。因此,Zn||最佳 ZSO-ChBr 电解质中的 Zn 对称电池在 5 mA cm-2 和 1 mAh cm-2 下可以获得超过 6000 小时的显着使用寿命,此外,在 Zn||Cu 不对称电池。此外,Zn||MnO2 全电池表现出优异的倍率能力和循环稳定性,循环 400 次后容量保持率为 80%。这项工作提供了一种电解质工程的集成策略,以提升 Zn2+ 在负极-电解质界面的脱溶剂动力学,增强 Zn 负极的循环稳定性,有效改善水系锌离子电池 (AZIBs) 的电化学性能,促进各种储能系统的发展。
更新日期:2024-10-28
中文翻译:

卤化溶剂化结构和优选的 Zn (002) 通过痕量添加剂沉积,以实现水性锌离子电池的高可逆性
水系锌离子电池 (AZIB) 的正极材料已经取得了巨大的进展,但其实际应用受到 Zn 负极循环稳定性差的阻碍。本文将两亲性胆碱溴 (ChBr) 作为添加剂引入高浓度 ZnSO4 水性电解质中,不仅调节了传统的 Zn2+ 溶剂化结构,而且在电解质-阳极界面建立了静电屏蔽层。与原始的 3 M ZnSO4 电解质相比,ChBr 改性的 ZnSO4 电解质 (ZSO-ChBr) 被证明可有效促进镀锌/剥离的可逆性和 Zn (002) 平面的优先生长,以及抑制 Zn 负极表面的析氢和副反应。因此,Zn||最佳 ZSO-ChBr 电解质中的 Zn 对称电池在 5 mA cm-2 和 1 mAh cm-2 下可以获得超过 6000 小时的显着使用寿命,此外,在 Zn||Cu 不对称电池。此外,Zn||MnO2 全电池表现出优异的倍率能力和循环稳定性,循环 400 次后容量保持率为 80%。这项工作提供了一种电解质工程的集成策略,以提升 Zn2+ 在负极-电解质界面的脱溶剂动力学,增强 Zn 负极的循环稳定性,有效改善水系锌离子电池 (AZIBs) 的电化学性能,促进各种储能系统的发展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号