当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
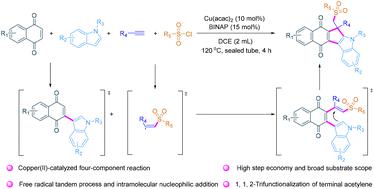
Copper(II)-catalyzed synthesis of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles via four-component cascade annulation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01560f Hong Xu, Jie Liao, Fei Ren, Chuan-Rong Zhou, Xiao-Zhuo Liu, Yao Xiao, Dong-Wei Hang, Fuyu Li, Bei Wang, Ji-Yu Wang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01560f Hong Xu, Jie Liao, Fei Ren, Chuan-Rong Zhou, Xiao-Zhuo Liu, Yao Xiao, Dong-Wei Hang, Fuyu Li, Bei Wang, Ji-Yu Wang
|
A copper-catalyzed four-component cascade annulation is realized to access novel sulfonyl-functionalized quinone-fused cyclopenta[b]indoles with high step economy and broad substrate scope, and 34 examples are constructed in one pot with up to 88% yield. This process includes sulfonyl radical triggered tandem cyclization by selective 1,1,2-trifunctionalization of terminal acetylene.
中文翻译:

铜 (II) 催化通过四组分级级环环环戊 [b] 吲哚合成
实现了铜催化的四组分级联环化,以获得新型磺酰官能化醌熔合环戊[b]吲哚,具有高步骤经济性和广泛的衬底范围,并在一个罐中构建了 34 个实例,产率高达 88%。该过程包括通过末端乙炔的选择性 1,1,2-三官能化实现磺酰自由基触发的串联环化。
更新日期:2024-10-29
中文翻译:

铜 (II) 催化通过四组分级级环环环戊 [b] 吲哚合成
实现了铜催化的四组分级联环化,以获得新型磺酰官能化醌熔合环戊[b]吲哚,具有高步骤经济性和广泛的衬底范围,并在一个罐中构建了 34 个实例,产率高达 88%。该过程包括通过末端乙炔的选择性 1,1,2-三官能化实现磺酰自由基触发的串联环化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号