当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile single-step synthesis of pentaaryl-substituted pyrano[3,2-b]pyrrol-5(1H)-ones showing aggregation-induced emission
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01600a Yongfeng Wang, Ruijun Chu, Yanxiao Wang, Mengyuan Zhou, Yuan Chen, Cheng Xu, Jingyu Sun, Guodong Yin
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01600a Yongfeng Wang, Ruijun Chu, Yanxiao Wang, Mengyuan Zhou, Yuan Chen, Cheng Xu, Jingyu Sun, Guodong Yin
|
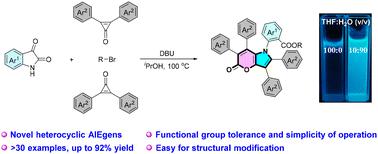
The continuing desire for multifunctional AIE materials has stimulated intensified research efforts to develop both innovative synthetic techniques and novel scaffolds. Herein, we report a new class of heterocyclic AIEgens, 1,2,3,6,7-pentaaryl substituted pyrano[3,2-b]pyrrol-5(1H)-ones, which contain an intriguing fused pyrrole/α-pyrone core. These structurally novel molecules were efficiently constructed via multicomponent reactions of easily accessible isatins, 2,3-diaryl cyclopropenones, and alkyl bromides in a single step under metal-free conditions. Subsequent late-stage modification of the resulting products was performed via the Suzuki–Miyaura coupling and reduction reactions. Furthermore, the HOMO/LUMO gaps were calculated and the single crystal structural analyses were conducted in order to gain deeper insights into the photophysical properties of these propeller-like AIEgens.
中文翻译:

五芳基取代的吡喃[3,2-b]吡咯-5(1H)-酮的简便单步合成显示聚集诱导的发射
对多功能 AIE 材料的持续需求激发了加大研究力度,以开发创新的合成技术和新型支架。在此,我们报道了一类新的杂环 AIEgens,1,2,3,6,7-戊酰取代的吡喃[3,2-b]吡咯-5(1H)-酮,它包含一个有趣的熔融吡咯/α-吡喃酮核心。在无金属条件下,通过易接近的蚂蚁素、2,3-二芳基环丙烯酮和烷基溴的多组分反应,一步有效地构建了这些结构新颖的分子。随后通过 Suzuki-Miyaura 偶联和还原反应对所得产物进行后期修饰。此外,计算了 HOMO/LUMO 间隙并进行了单晶结构分析,以便更深入地了解这些类似螺旋桨的 AIEgens 的光物理特性。
更新日期:2024-10-29
中文翻译:

五芳基取代的吡喃[3,2-b]吡咯-5(1H)-酮的简便单步合成显示聚集诱导的发射
对多功能 AIE 材料的持续需求激发了加大研究力度,以开发创新的合成技术和新型支架。在此,我们报道了一类新的杂环 AIEgens,1,2,3,6,7-戊酰取代的吡喃[3,2-b]吡咯-5(1H)-酮,它包含一个有趣的熔融吡咯/α-吡喃酮核心。在无金属条件下,通过易接近的蚂蚁素、2,3-二芳基环丙烯酮和烷基溴的多组分反应,一步有效地构建了这些结构新颖的分子。随后通过 Suzuki-Miyaura 偶联和还原反应对所得产物进行后期修饰。此外,计算了 HOMO/LUMO 间隙并进行了单晶结构分析,以便更深入地了解这些类似螺旋桨的 AIEgens 的光物理特性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号