当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective access to furan-fused [5.5.0] bicyclic heterocycles enabled by gold-catalyzed asymmetric [8 + 4] cycloaddition
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01841a Xunhua Wang, Jianhua Wang, Xiaoxun Li
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-29 , DOI: 10.1039/d4qo01841a Xunhua Wang, Jianhua Wang, Xiaoxun Li
|
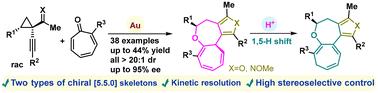
The construction of fused [5.5.0] bicyclic heterocycles with precise regio-, stereo-, and enantioselective control remains a significant challenge in asymmetric catalysis. In this work, we introduce a novel gold-catalyzed asymmetric [8 + 4] cycloaddition reaction of 1-(1-alkynyl)cyclopropyl ketones with simple tropones, yielding highly functionalized cyclohepta[b]furo[3,4-d]oxepine derivatives with excellent diastereo- and enantioselectivity (38 examples, all >20 : 1 dr, up to 95% ee). Additionally, an efficient kinetic resolution (KR) process is achieved (s factor up to 104). The gram-scale synthesis and subsequent synthetic transformations of the cycloadducts further demonstrate the synthetic potential of this method. Furthermore, the cycloadduct can undergo a [1,5]-H shift under acid catalysis, adding another dimension to the structural diversity.
中文翻译:

通过金催化的不对称 [8 + 4] 环加成实现对呋喃融合 [5.5.0] 双环杂环的立体选择性访问
构建具有精确区域、立体和对映选择性控制的熔融 [5.5.0] 双环杂环仍然是不对称催化中的一个重大挑战。在这项工作中,我们介绍了 1-(1-炔基)环丙基酮与简单托品的新型金催化不对称 [8 + 4] 环加成反应,产生了高度功能化的环庚[b]呋喃[3,4-d]氧嘌平衍生物,具有优异的非对映选择性和对映选择性(38 个实例,均为 >20 : 1 dr,高达 95% ee)。此外,还实现了高效的动力学分辨率 (KR) 过程(s 因子高达 104)。环加合物的克级合成和随后的合成转化进一步证明了该方法的合成潜力。此外,环加合物可以在酸催化下发生 [1,5]-H 偏移,为结构多样性增加了另一个维度。
更新日期:2024-10-29
中文翻译:

通过金催化的不对称 [8 + 4] 环加成实现对呋喃融合 [5.5.0] 双环杂环的立体选择性访问
构建具有精确区域、立体和对映选择性控制的熔融 [5.5.0] 双环杂环仍然是不对称催化中的一个重大挑战。在这项工作中,我们介绍了 1-(1-炔基)环丙基酮与简单托品的新型金催化不对称 [8 + 4] 环加成反应,产生了高度功能化的环庚[b]呋喃[3,4-d]氧嘌平衍生物,具有优异的非对映选择性和对映选择性(38 个实例,均为 >20 : 1 dr,高达 95% ee)。此外,还实现了高效的动力学分辨率 (KR) 过程(s 因子高达 104)。环加合物的克级合成和随后的合成转化进一步证明了该方法的合成潜力。此外,环加合物可以在酸催化下发生 [1,5]-H 偏移,为结构多样性增加了另一个维度。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号