当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
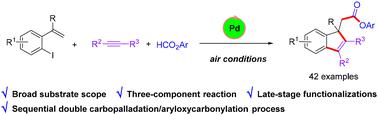
Palladium-catalyzed synthesis of indene-1-acetates via sequential double carbopalladation and aryloxycarbonylation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-28 , DOI: 10.1039/d4qo01570c Fei Sun, Yiyi Zheng, Zhongyao Jiang, Mingxia Wu, Zeng Lv, Hongsen Ji, Xin-Xing Wu
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-28 , DOI: 10.1039/d4qo01570c Fei Sun, Yiyi Zheng, Zhongyao Jiang, Mingxia Wu, Zeng Lv, Hongsen Ji, Xin-Xing Wu
|
A novel palladium-catalyzed cascade reaction of o-iodostyrenes, internal alkynes and formates to access various indene-1-acetates was developed. This method enables the construction of three C–C bonds and one C–O bond through sequential double carbopalladation and aryloxycarbonylation under a palladium/air system. This protocol exhibits high regioselectivity and wide functional group tolerance. In addition, the synthetic utility of this protocol has been successfully demonstrated by the gram-scale synthesis and the late-stage modification of a series of complex bioactive molecules.
中文翻译:

钯催化通过连续双碳钂化和芳氧基羰基化合成茚-1-乙酸酯
开发了一种新型钯催化的邻碘苯乙烯、内炔烃和甲酸盐级联反应,以获得各种茚-1-乙酸盐。该方法能够在钯/空气体系下通过连续的双碳球化和芳氧基羰基化构建三个 C-C 键和一个 C-O 键。该方案表现出高区域选择性和广泛的官能团耐受性。此外,该方案的合成效用已通过一系列复杂生物活性分子的克级合成和后期修饰成功证明。
更新日期:2024-11-01
中文翻译:

钯催化通过连续双碳钂化和芳氧基羰基化合成茚-1-乙酸酯
开发了一种新型钯催化的邻碘苯乙烯、内炔烃和甲酸盐级联反应,以获得各种茚-1-乙酸盐。该方法能够在钯/空气体系下通过连续的双碳球化和芳氧基羰基化构建三个 C-C 键和一个 C-O 键。该方案表现出高区域选择性和广泛的官能团耐受性。此外,该方案的合成效用已通过一系列复杂生物活性分子的克级合成和后期修饰成功证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号