当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C–H acylation as an enabling tool to tag phenolic drugs
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-28 , DOI: 10.1039/d4qo01505c Carlota Girón-Elola, Arkaitz Correa
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-28 , DOI: 10.1039/d4qo01505c Carlota Girón-Elola, Arkaitz Correa
|
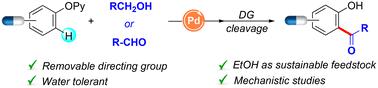
The site-selective functionalization of value-added compounds while implementing atom-economical C–H coupling partners represents an unmet challenge of utmost importance within organic synthesis. Herein, we report a Pd-catalyzed directed C–H acylation of a collection of relevant phenol-containing compounds with ethanol and other alcohols and aldehydes. This tagging technique is distinguished by its water compatibility and predictable regioselectivity and features the use of ethanol as renewable feedstock for the modification of intricate phenols, including estrogens and other top-selling pharmaceuticals. Mechanistic studies support the intermediacy of a challenging 6-membered dimeric palladacycle that undergoes the addition of nucleophilic acyl radical species.
中文翻译:

C-H 酰化作为标记酚类药物的使能工具
在实施原子经济的 C-H 偶联伴侣的同时,增值化合物的位点选择性官能化代表了有机合成中一个尚未解决的极其重要的挑战。在此,我们报道了一组相关含酚化合物与乙醇和其他醇和醛的 Pd 催化定向 C-H 酰化。这种标记技术以其水相容性和可预测的区域选择性而著称,其特点是使用乙醇作为可再生原料来修饰复杂的酚类,包括雌激素和其他最畅销的药物。机制研究支持具有挑战性的 6 元二聚体 palladacycle 的中介性,该环经历了亲核酰基自由基物种的添加。
更新日期:2024-10-29
中文翻译:

C-H 酰化作为标记酚类药物的使能工具
在实施原子经济的 C-H 偶联伴侣的同时,增值化合物的位点选择性官能化代表了有机合成中一个尚未解决的极其重要的挑战。在此,我们报道了一组相关含酚化合物与乙醇和其他醇和醛的 Pd 催化定向 C-H 酰化。这种标记技术以其水相容性和可预测的区域选择性而著称,其特点是使用乙醇作为可再生原料来修饰复杂的酚类,包括雌激素和其他最畅销的药物。机制研究支持具有挑战性的 6 元二聚体 palladacycle 的中介性,该环经历了亲核酰基自由基物种的添加。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号