当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discrimination between OH− and H2O oxidation for oxygen evolution reaction
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.checat.2024.101157 Mengjun Xiao, Qianbao Wu, Hongfei Liu, Xia Zheng, Lei Li, Wei Wang, Chunhua Cui
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-28 , DOI: 10.1016/j.checat.2024.101157 Mengjun Xiao, Qianbao Wu, Hongfei Liu, Xia Zheng, Lei Li, Wei Wang, Chunhua Cui
|
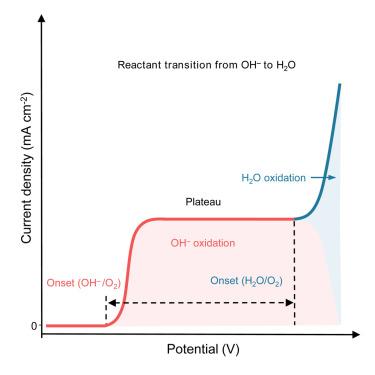
OH− 2 O-reactant discrimination for the oxygen evolution reaction (OER) is a critical but not well resolved issue. This has led to unreasonable activity comparisons, misinterpreted OER mechanisms, and ununified models for theoretical calculations regardless of the thermodynamic/kinetic difference between OH− 2 O oxidation. Here, we discriminate between OH− 2 O oxidation by tuning the interfacial OH− in situ 16 OH− 2 18 O isotopic labeling-based differential electrochemical mass spectrometry, we examine the respective electrochemical oxidation behaviors between OH− 2 O oxidation. We find that OH− 2 O oxidation and that Tafel plotting gives slopes of ∼50 mV dec−1 for OH− −1 for H2 O oxidation on a model CoOOH catalyst. This work calls for the discrimination of OH− 2 O oxidation as the prerequisite for future OER activity evaluation and mechanism studies.
中文翻译:

析氧反应中 OH− 和 H2O 氧化的区分
析氧反应 (OER) 的 OH − / H 2 O 反应物区分是一个关键但尚未得到充分解决的问题。这导致了不合理的活性比较、对 OER 机制的误解以及不统一的理论计算模型,而不管 OH− 和 H2O 氧化之间的热力学/动力学差异如何。在这里,我们通过调整界面 OH− 浓度来区分 OH− 和 H2O 氧化。将 OER 动力学分析与基于原位 16OH − / H218O 同位素标记的差分电化学质谱相结合,我们研究了 OH − 和 H 2 O 氧化之间各自的电化学氧化行为。我们发现,OH− 氧化相对于 H2O 氧化的起始电位低 ∼550 mV,并且塔菲尔图给出 OH− 氧化的斜率为 ∼50 mV dec-1,大大低于模型 CoOOH 催化剂上 H2O 氧化的 ∼200 mV dec-1。这项工作要求区分 OH−/H2O 氧化作为未来 OER 活性评估和机制研究的先决条件。
更新日期:2024-10-28
中文翻译:

析氧反应中 OH− 和 H2O 氧化的区分
析氧反应 (OER) 的 OH − / H 2 O 反应物区分是一个关键但尚未得到充分解决的问题。这导致了不合理的活性比较、对 OER 机制的误解以及不统一的理论计算模型,而不管 OH− 和 H2O 氧化之间的热力学/动力学差异如何。在这里,我们通过调整界面 OH− 浓度来区分 OH− 和 H2O 氧化。将 OER 动力学分析与基于原位 16OH − / H218O 同位素标记的差分电化学质谱相结合,我们研究了 OH − 和 H 2 O 氧化之间各自的电化学氧化行为。我们发现,OH− 氧化相对于 H2O 氧化的起始电位低 ∼550 mV,并且塔菲尔图给出 OH− 氧化的斜率为 ∼50 mV dec-1,大大低于模型 CoOOH 催化剂上 H2O 氧化的 ∼200 mV dec-1。这项工作要求区分 OH−/H2O 氧化作为未来 OER 活性评估和机制研究的先决条件。

































 京公网安备 11010802027423号
京公网安备 11010802027423号