当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salt Disproportionation in the Solid State: Role of Solubility and Counterion Volatility
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2016-11-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.6b00745 Naveen K. Thakral 1, 2 , Robert J. Behme 1 , Aktham Aburub 1 , Jeffrey A. Peterson 1 , Timothy A. Woods 1 , Benjamin A. Diseroad 1 , Raj Suryanarayanan 2 , Gregory A. Stephenson 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2016-11-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.6b00745 Naveen K. Thakral 1, 2 , Robert J. Behme 1 , Aktham Aburub 1 , Jeffrey A. Peterson 1 , Timothy A. Woods 1 , Benjamin A. Diseroad 1 , Raj Suryanarayanan 2 , Gregory A. Stephenson 1
Affiliation
|
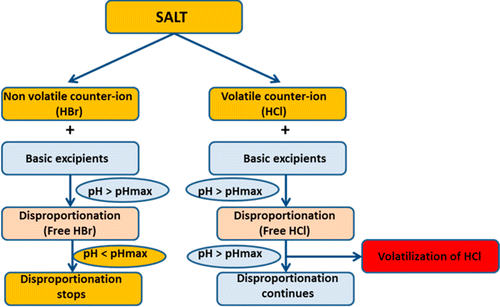
Disproportionation propensity of salts (HCl, HBr, heminapadisylate) and adipic acid cocrystal of corticotropin releasing hormone receptor-1 antagonist was studied using model free kinetics. Using thermogravimetic weight loss profile or heat flow curves from differential scanning calorimetry, an activation energy plot for salts and cocrystal was generated based on model free kinetics. This activation energy of disproportionation provided qualitative information about the solid state salt stability. To ensure the stability throughout the shelf life, “prototype” formulations of salts and cocrystal in tablet form were stored at 40 °C and several water vapor pressures. Disproportionation kinetics were studied in these prototype tablet formulations using two-dimensional X-ray diffractometry. Formulations containing the adipic acid cocrystal or heminapadisylate salt did not show disproportionation of API when stored at 40 °C/75% RH for 300 days. On the other hand, formulations containing HCl or HBr salt disproportionated. Though isostructural, the disproportionation propensity of HBr and HCl salts was quite different. The HCl salt highlighted the important role that volatility of the counterion plays in the physical stability of the formulations. Solution state stability (i.e., in dissolution medium) of salts and cocrystal was also assessed and compared with solid state stability, by determining their solubility at different pH’s, and intrinsic dissolution rate.
中文翻译:

固态中的盐歧化:溶解度和抗衡离子挥发性的作用
使用模型自由动力学研究了促肾上腺皮质激素释放激素受体-1拮抗剂的盐(HCl,HBr,半氨二甲酸酯)和己二酸共晶体的歧化倾向。使用热重重量损失曲线或差示扫描量热法的热流曲线,基于模型自由动力学生成了盐和共晶的活化能图。这种歧化作用的活化能提供了有关固态盐稳定性的定性信息。为了确保在整个货架期内的稳定性,将盐和共晶片剂的“原型”配方以40℃和数个水蒸气压力存储。使用二维X射线衍射法研究了这些原型片剂中的歧化动力学。当在40°C / 75%RH下保存300天时,含有己二酸共晶体或半氨基二甲磺酸盐的制剂未显示API歧化。另一方面,含有HCl或HBr盐的配方不成比例。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。
更新日期:2016-11-02
中文翻译:

固态中的盐歧化:溶解度和抗衡离子挥发性的作用
使用模型自由动力学研究了促肾上腺皮质激素释放激素受体-1拮抗剂的盐(HCl,HBr,半氨二甲酸酯)和己二酸共晶体的歧化倾向。使用热重重量损失曲线或差示扫描量热法的热流曲线,基于模型自由动力学生成了盐和共晶的活化能图。这种歧化作用的活化能提供了有关固态盐稳定性的定性信息。为了确保在整个货架期内的稳定性,将盐和共晶片剂的“原型”配方以40℃和数个水蒸气压力存储。使用二维X射线衍射法研究了这些原型片剂中的歧化动力学。当在40°C / 75%RH下保存300天时,含有己二酸共晶体或半氨基二甲磺酸盐的制剂未显示API歧化。另一方面,含有HCl或HBr盐的配方不成比例。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。尽管是同构的,但HBr和HCl盐的歧化倾向却大不相同。HCl盐突出了抗衡离子的挥发性在制剂的物理稳定性中发挥的重要作用。还通过确定盐和共晶在不同pH值下的溶解度和固有溶解速率,来评估盐和共晶的溶液状态稳定性(即在溶解介质中),并将其与固态稳定性进行比较。















































 京公网安备 11010802027423号
京公网安备 11010802027423号