当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical and modular cycloadditions of in-situ formed exocyclic vinylcarbenes
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-25 , DOI: 10.1016/j.checat.2024.101163 Cheng Zhang, Shanliang Dong, Martin C. Dietl, Matthias Rudolph, Xinke Zhang, Kemiao Hong, Wei Yi, A. Stephen K. Hashmi, Xinfang Xu
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-10-25 , DOI: 10.1016/j.checat.2024.101163 Cheng Zhang, Shanliang Dong, Martin C. Dietl, Matthias Rudolph, Xinke Zhang, Kemiao Hong, Wei Yi, A. Stephen K. Hashmi, Xinfang Xu
|
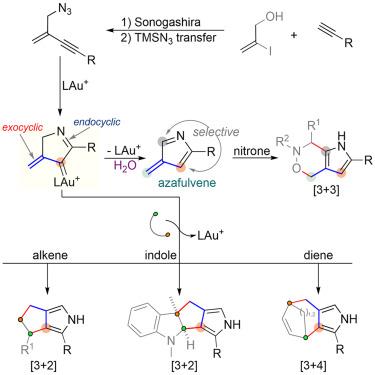
The exploration of reactive intermediates, which enable chemo- and regioselective cycloaddition reactions for the expeditious construction of fused and/or bridged ring systems, continues to draw a great deal of interest from the synthetic community. Vinylcarbene species, which serve as 3-carbon building blocks, have been frequently used for the construction of (hetero)cyclic frameworks through the successive formation of multiple carbon–carbon and/or carbon–heteroatom bonds. Herein, we report a concise strategy for the catalytic generation of an exocyclic α-vinyl gold carbene species via a selective gold(I)-promoted azide-enyne cyclization process. Subsequently, practical and modular cycloadditions of these in-situ-formed intermediates with different types of partners were disclosed, producing a diverse array of fused and bridged pyrroles in high chemo-, regio-, and stereoselectivity.
中文翻译:

原位形成的外环乙烯基卡宾的实用和模块化环加成反应
反应性中间体的探索使化学和区域选择性环加成反应能够快速构建融合和/或桥接环系统,继续引起合成界的极大兴趣。乙烯基卡宾物种作为 3 碳结构单元,通过连续形成多个碳-碳和/或碳-杂原子键,经常用于构建(杂)环框架。在此,我们报道了一种通过选择性金 (I) 促进的叠氮化物-烯炔环化过程催化生成外环 α-乙烯基金卡宾物种的简洁策略。随后,这些原位形成的中间体与不同类型的伙伴的实用和模块化环加成反应被公开,产生了一系列具有高化学选择性、区域选择性和立体选择性的多种融合和桥接吡咯。
更新日期:2024-10-25
中文翻译:

原位形成的外环乙烯基卡宾的实用和模块化环加成反应
反应性中间体的探索使化学和区域选择性环加成反应能够快速构建融合和/或桥接环系统,继续引起合成界的极大兴趣。乙烯基卡宾物种作为 3 碳结构单元,通过连续形成多个碳-碳和/或碳-杂原子键,经常用于构建(杂)环框架。在此,我们报道了一种通过选择性金 (I) 促进的叠氮化物-烯炔环化过程催化生成外环 α-乙烯基金卡宾物种的简洁策略。随后,这些原位形成的中间体与不同类型的伙伴的实用和模块化环加成反应被公开,产生了一系列具有高化学选择性、区域选择性和立体选择性的多种融合和桥接吡咯。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号