Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting cryo-EM structures of actomyosin-5a to reveal the physical properties of its lever
Structure ( IF 4.4 ) Pub Date : 2024-10-24 , DOI: 10.1016/j.str.2024.09.025 Molly S.C. Gravett, David P. Klebl, Oliver G. Harlen, Daniel J. Read, Stephen P. Muench, Sarah A. Harris, Michelle Peckham
Structure ( IF 4.4 ) Pub Date : 2024-10-24 , DOI: 10.1016/j.str.2024.09.025 Molly S.C. Gravett, David P. Klebl, Oliver G. Harlen, Daniel J. Read, Stephen P. Muench, Sarah A. Harris, Michelle Peckham
|
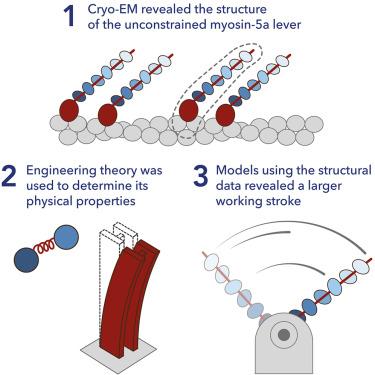
Myosin 5a (Myo5a) is a dimeric processive motor protein that transports cellular cargos along filamentous actin (F-actin). Its long lever is responsible for its large power-stroke, step size, and load-bearing ability. Little is known about the levers’ structure and physical properties, and how they contribute to walking mechanics. Using cryoelectron microscopy (cryo-EM) and molecular dynamics (MD) simulations, we resolved the structure of monomeric Myo5a, comprising the motor domain and full-length lever, bound to F-actin. The range of its lever conformations revealed its physical properties, how stiffness varies along its length and predicts a large, 35 nm, working stroke. Thus, the newly released trail head in a dimeric Myo5a would only need to perform a small diffusive search for its new binding site on F-actin, and stress would only be generated across the dimer once phosphate is released from the lead head, revealing new insight into the walking behavior of Myo5a.
中文翻译:

利用肌动球蛋白-5a 的冷冻电镜结构来揭示其杠杆的物理特性
肌球蛋白 5a (Myo5a) 是一种二聚体进行性运动蛋白,可沿丝状肌动蛋白 (F-肌动蛋白) 运输细胞货物。它的长杆负责其较大的功率冲程、步长和承载能力。人们对杠杆的结构和物理特性以及它们如何影响行走机制知之甚少。使用冷冻电子显微镜 (cryo-EM) 和分子动力学 (MD) 模拟,我们解析了与 F-肌动蛋白结合的单体 Myo5a 的结构,包括运动域和全长杠杆。它的杠杆构象范围揭示了它的物理特性,刚度如何沿其长度变化,并预测了一个 35 nm 的大工作行程。因此,二聚体 Myo5a 中新释放的尾迹头只需要对其在 F-肌动蛋白上的新结合位点进行小规模的扩散搜索,并且只有在磷酸盐从铅头释放后才会在二聚体上产生应力,揭示了对 Myo5a 行走行为的新见解。
更新日期:2024-10-24
中文翻译:

利用肌动球蛋白-5a 的冷冻电镜结构来揭示其杠杆的物理特性
肌球蛋白 5a (Myo5a) 是一种二聚体进行性运动蛋白,可沿丝状肌动蛋白 (F-肌动蛋白) 运输细胞货物。它的长杆负责其较大的功率冲程、步长和承载能力。人们对杠杆的结构和物理特性以及它们如何影响行走机制知之甚少。使用冷冻电子显微镜 (cryo-EM) 和分子动力学 (MD) 模拟,我们解析了与 F-肌动蛋白结合的单体 Myo5a 的结构,包括运动域和全长杠杆。它的杠杆构象范围揭示了它的物理特性,刚度如何沿其长度变化,并预测了一个 35 nm 的大工作行程。因此,二聚体 Myo5a 中新释放的尾迹头只需要对其在 F-肌动蛋白上的新结合位点进行小规模的扩散搜索,并且只有在磷酸盐从铅头释放后才会在二聚体上产生应力,揭示了对 Myo5a 行走行为的新见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号