当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of ABCG2 Transporter Activity by Ko143 Derivatives
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-10-24 , DOI: 10.1021/acschembio.4c00353
Qin Yu 1 , Sepehr Dehghani-Ghahnaviyeh 2 , Ali Rasouli 2 , Anna Sadurni 3 , Julia Kowal 1 , Rose Bang-Soerensen 1 , Po-Chao Wen 2 , Melanie Tinzl-Zechner 3 , Rossitza N Irobalieva 1 , Dongchun Ni 4 , Henning Stahlberg 4 , Karl-Heinz Altmann 3 , Emad Tajkhorshid 2 , Kaspar P Locher 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-10-24 , DOI: 10.1021/acschembio.4c00353
Qin Yu 1 , Sepehr Dehghani-Ghahnaviyeh 2 , Ali Rasouli 2 , Anna Sadurni 3 , Julia Kowal 1 , Rose Bang-Soerensen 1 , Po-Chao Wen 2 , Melanie Tinzl-Zechner 3 , Rossitza N Irobalieva 1 , Dongchun Ni 4 , Henning Stahlberg 4 , Karl-Heinz Altmann 3 , Emad Tajkhorshid 2 , Kaspar P Locher 1
Affiliation
|
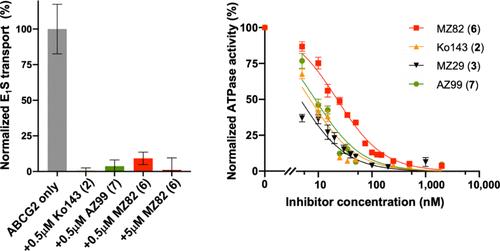
ABCG2 is a multidrug transporter that protects tissues from xenobiotics, affects drug pharmacokinetics, and contributes to multidrug resistance of cancer cells. Here, we present tetracyclic fumitremorgin C analog Ko143 derivatives, evaluate their in vitro modulation of purified ABCG2, and report four high-resolution cryo-EM structures and computational analyses to elucidate their interactions with ABCG2. We found that Ko143 derivatives that are based on a ring-opened scaffold no longer inhibit ABCG2-mediated transport activity. In contrast, closed-ring, tetracyclic analogs were highly potent inhibitors. Strikingly, the least potent of these compounds, MZ82, bound deeper into the central ABCG2 cavity than the other inhibitors and it led to partial closure of the transmembrane domains and increased flexibility of the nucleotide-binding domains. Minor structural modifications can thus convert a potent inhibitor into a compound that induces conformational changes in ABCG2 similar to those observed during binding of a substrate. Molecular dynamics simulations and free energy binding calculations further supported the correlation between reduced potency and distinct binding pose of the compounds. We introduce the highly potent inhibitor AZ99 that may exhibit improved in vivo stability.
中文翻译:

Ko143 衍生物对 ABCG2 转运蛋白活性的调节
ABCG2 是一种多药转运蛋白,可保护组织免受外源性物质的侵害,影响药物药代动力学,并有助于癌细胞的多药耐药性。在这里,我们提出了四环烟曲霉菌素 C 类似物 Ko143 衍生物,评估了它们对纯化的 ABCG2 的体外调节,并报告了四种高分辨率冷冻电镜结构和计算分析,以阐明它们与 ABCG2 的相互作用。我们发现基于开环支架的 Ko143 衍生物不再抑制 ABCG2 介导的转运活动。相比之下,闭环四环类似物是强效抑制剂。引人注目的是,这些化合物中效力最弱的 MZ82 比其他抑制剂更深地结合到中央 ABCG2 腔中,它导致跨膜结构域部分闭合并增加核苷酸结合结构域的柔韧性。因此,微小的结构修饰可以将有效的抑制剂转化为化合物,诱导 ABCG2 的构象变化,类似于在底物结合过程中观察到的变化。分子动力学模拟和自由能结合计算进一步支持化合物效力降低与不同结合姿势之间的相关性。我们介绍了高效的抑制剂 AZ99,它可能表现出更好的体内稳定性。
更新日期:2024-10-24
中文翻译:

Ko143 衍生物对 ABCG2 转运蛋白活性的调节
ABCG2 是一种多药转运蛋白,可保护组织免受外源性物质的侵害,影响药物药代动力学,并有助于癌细胞的多药耐药性。在这里,我们提出了四环烟曲霉菌素 C 类似物 Ko143 衍生物,评估了它们对纯化的 ABCG2 的体外调节,并报告了四种高分辨率冷冻电镜结构和计算分析,以阐明它们与 ABCG2 的相互作用。我们发现基于开环支架的 Ko143 衍生物不再抑制 ABCG2 介导的转运活动。相比之下,闭环四环类似物是强效抑制剂。引人注目的是,这些化合物中效力最弱的 MZ82 比其他抑制剂更深地结合到中央 ABCG2 腔中,它导致跨膜结构域部分闭合并增加核苷酸结合结构域的柔韧性。因此,微小的结构修饰可以将有效的抑制剂转化为化合物,诱导 ABCG2 的构象变化,类似于在底物结合过程中观察到的变化。分子动力学模拟和自由能结合计算进一步支持化合物效力降低与不同结合姿势之间的相关性。我们介绍了高效的抑制剂 AZ99,它可能表现出更好的体内稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号