当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy of prediction rule and total synthesis in solving the stereochemical puzzle of eucalactam B
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-24 , DOI: 10.1039/d4qo01777c Chenqi Wang, Junyang Liu, Tao Ye
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-24 , DOI: 10.1039/d4qo01777c Chenqi Wang, Junyang Liu, Tao Ye
|
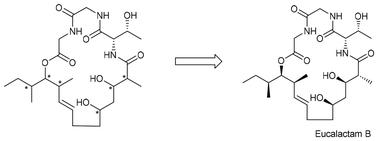
The absolute configurations of the fungal-derived reduced polyketide eucalactam B were initially predicted using the “Biochemistry-based rule” and later confirmed through its first successful total synthesis. This accomplishment involved key reactions such as Brown crotylation, the Paterson anti-aldol reaction, cross-metathesis, and macrolactamization, furnishing eucalactam B in 19 linear steps, with an overall yield of 6.0%. The high degree of alignment between the synthetic compound and the natural product provided strong validation for the accuracy of the “Biochemistry-based rule” in predicting the absolute configurations of fungal-derived reduced polyketides.
中文翻译:

预测规则与全合成的协同作用解决桉卡坦 B 立体化学难题
真菌衍生的还原型聚酮桉肌酰胺 B 的绝对构型最初使用“基于生物化学的规则”进行预测,后来通过其首次成功的全合成得到证实。这一成就涉及关键反应,如 Brown crotylation、Paterson 抗羟醛反应、交叉复分解和大内酰胺化,以 19 个线性步骤提供桉卡坦 B,总产率为 6.0%。合成化合物和天然产物之间的高度对齐为“基于生物化学的规则”在预测真菌衍生的还原聚酮的绝对构型方面的准确性提供了有力的验证。
更新日期:2024-10-24
中文翻译:

预测规则与全合成的协同作用解决桉卡坦 B 立体化学难题
真菌衍生的还原型聚酮桉肌酰胺 B 的绝对构型最初使用“基于生物化学的规则”进行预测,后来通过其首次成功的全合成得到证实。这一成就涉及关键反应,如 Brown crotylation、Paterson 抗羟醛反应、交叉复分解和大内酰胺化,以 19 个线性步骤提供桉卡坦 B,总产率为 6.0%。合成化合物和天然产物之间的高度对齐为“基于生物化学的规则”在预测真菌衍生的还原聚酮的绝对构型方面的准确性提供了有力的验证。































 京公网安备 11010802027423号
京公网安备 11010802027423号