当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrrolylsulfonium salts: stable, accessible and versatile pseudohalides for Stille couplings
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-24 , DOI: 10.1039/d4qo01793e Jodie L. Hann, Catherine L. Lyall, Gabriele Kociok-Köhn, Simon E. Lewis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-24 , DOI: 10.1039/d4qo01793e Jodie L. Hann, Catherine L. Lyall, Gabriele Kociok-Köhn, Simon E. Lewis
|
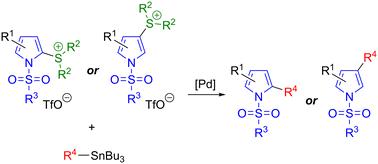
Pyrrolyl halides can be difficult to synthesise in a regioselective manner and are often unstable, which has hampered their application in cross-coupling. Here we introduce pyrrolylsulfonium salts as advantageous pseudohalide coupling partners and showcase their applicability in Stille couplings. Benefits of these salts include their straightforward synthesis via an “interrupted Pummerer” process, their high stability, and the ability to selectively introduce the sulfonium group at either the pyrrole α- or β- position as required. The Stille coupling has been demonstrated for aryl, heteroaryl and alkynylstannanes, and the effect of the pyrrole substituents on the regioselectivity of S–C bond activation has been investigated. Conditions to effect N-desulfonylation of N-trisyl coupling products have been identified.
中文翻译:

吡咯基磺盐:稳定、可接近且用途广泛的伪卤化物,用于 Stille 偶联
吡咯酰卤化物很难以区域选择性方式合成,并且通常不稳定,这阻碍了它们在交叉偶联中的应用。在这里,我们介绍了吡咯基磺盐作为有利的伪卤化物偶联伙伴,并展示了它们在 Stille 偶联中的适用性。这些盐的优点包括通过“间断 Pummerer”工艺直接合成、高稳定性以及能够根据需要选择性地在吡咯 α 或 β 位置引入磺基团。已经证明了芳基、杂芳基和炔基锡烷的 Stille 偶联,并且已经研究了吡咯取代基对 S-C 键活化区域选择性的影响。已经确定了影响 N-三酰基偶联产物的 N-脱磺酰化的条件。
更新日期:2024-10-25
中文翻译:

吡咯基磺盐:稳定、可接近且用途广泛的伪卤化物,用于 Stille 偶联
吡咯酰卤化物很难以区域选择性方式合成,并且通常不稳定,这阻碍了它们在交叉偶联中的应用。在这里,我们介绍了吡咯基磺盐作为有利的伪卤化物偶联伙伴,并展示了它们在 Stille 偶联中的适用性。这些盐的优点包括通过“间断 Pummerer”工艺直接合成、高稳定性以及能够根据需要选择性地在吡咯 α 或 β 位置引入磺基团。已经证明了芳基、杂芳基和炔基锡烷的 Stille 偶联,并且已经研究了吡咯取代基对 S-C 键活化区域选择性的影响。已经确定了影响 N-三酰基偶联产物的 N-脱磺酰化的条件。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号