当前位置:
X-MOL 学术
›
Am. J. Hematol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toxicities and outcome after CD19-directed chimeric antigen receptor T-cell therapy for secondary neurolymphomatosis
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-10-23 , DOI: 10.1002/ajh.27505 Leon D. Kaulen, Philipp Karschnia, Sofia Doubrovinskaia, Jeremy S. Abramson, Maria Martinez-Lage, Ganesh Shankar, Bryan D. Choi, Jeffrey A. Barnes, Areej El-Jawahri, Ephraim P. Hochberg, P. Connor Johnson, Jacob D. Soumerai, Wolfgang Wick, Marcela V. Maus, Yi-Bin Chen, Matthew J. Frigault, Jorg Dietrich
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-10-23 , DOI: 10.1002/ajh.27505 Leon D. Kaulen, Philipp Karschnia, Sofia Doubrovinskaia, Jeremy S. Abramson, Maria Martinez-Lage, Ganesh Shankar, Bryan D. Choi, Jeffrey A. Barnes, Areej El-Jawahri, Ephraim P. Hochberg, P. Connor Johnson, Jacob D. Soumerai, Wolfgang Wick, Marcela V. Maus, Yi-Bin Chen, Matthew J. Frigault, Jorg Dietrich
|
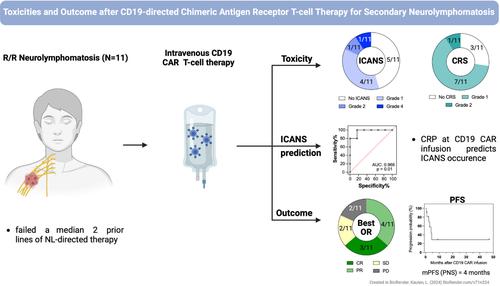
Lymphomatous infiltration of the peripheral nervous system (PNS), termed neurolymphomatosis, represents a distinct extranodal non-Hodgkin lymphoma variant with dismal outcome. CD19-directed chimeric antigen receptor (CD19-CAR) T-cell therapy has emerged as a safe and effective treatment for B-cell lymphomas. We aimed to assess toxicity and efficacy of CD19-CAR T-cells in neurolymphomatosis. Neurolymphomatosis patients treated with CD19 CAR T-cells were retrospectively identified at Massachusetts General Hospital over a six-year period. Toxicities were graded according to the ASTCT classification, management, and response rates were recorded. Eleven neurolymphomatosis patients were identified with a median of 2 lines of PNS-directed treatments (range: 1-3) prior to receiving CD19-CAR T-cells. Neurolymphomatosis localized to the nerve roots (8/11, 73%), plexus (5/11, 45%), peripheral (4/11, 36%) and cranial nerves (5/11, 45%). Low grade cytokine release syndrome (CRS) was detected in 8/11 (73%; grade 1: N = 7; grade 2: N = 1) cases. Low- and high-grade immune cell-associated neurotoxicity syndrome (ICANS) were recorded in 5/11 (45%; grade 1: N = 4; grade 2: N = 1) and 1/11 (9%; grade 4) patients, respectively. CRP levels at infusion were predictive of ICANS (area under the curve: 0.96, p = 0.01). Seven of eleven neurolymphomatosis patients (64%) responded to CD19-CAR T-cells. Complete remissions (CR) were achieved in three cases (27%), with 2 patients in sustained CR nine and 46 months after CD19-CAR infusion. Median progression-free survival (PFS) was 4 months. Collectively, CD19-CAR T-cell treatment was well tolerated and showed promising efficacy in recurrent neurolymphomatosis, a difficult to treat condition with unmet medical need. Findings suggest that CD19-CAR may sufficiently penetrate the blood-nerve barrier. Toxicity and outcomes were overall similar to CAR-T cell therapy in CNS lymphoma.
中文翻译:

CD19 定向嵌合抗原受体 T 细胞治疗继发性神经淋巴瘤病后的毒性和结局
周围神经系统 (PNS) 淋巴瘤浸润,称为神经淋巴瘤病,代表一种独特的结外非霍奇金淋巴瘤变体,结果令人沮丧。CD19 定向嵌合抗原受体 (CD19-CAR) T 细胞疗法已成为一种安全有效的 B 细胞淋巴瘤治疗方法。我们旨在评估 CD19-CAR T 细胞在神经淋巴瘤病中的毒性和疗效。在麻省总医院回顾性确定了接受 CD19 CAR T 细胞治疗的神经淋巴瘤病患者,为期 6 年。根据 ASTCT 分类对毒性进行分级,记录管理和反应率。在接受 CD19-CAR T 细胞之前,确定 11 例神经淋巴瘤病患者接受 2 线 PNS 定向治疗的中位数 (范围:1-3)。神经淋巴瘤病局限于神经根 (8/11, 73%)、神经丛 (5/11, 45%)、外周神经 (4/11, 36%) 和颅神经 (5/11, 45%)。在 8/11 (73%;1 级:N = 7;2 级:N = 1) 病例中检测到低级别细胞因子释放综合征 (CRS)。低级别和高级别免疫细胞相关神经毒性综合征 (ICANS) 分别见于 5/11 (45%;1 级:N = 4;2 级:N = 1) 和 1/11 (9%;4 级)患者。输注时 CRP 水平可预测 ICANS (曲线下面积: 0.96,p = 0.01)。11 例神经淋巴瘤病患者中有 7 例 (64%) 对 CD19-CAR T 细胞有反应。3 例 (27%) 达到完全缓解 (CR),其中 2 例患者在输注 CD19-CAR 后 9 个月和 46 个月持续 CR。中位无进展生存期 (PFS) 为 4 个月。总的来说,CD19-CAR T 细胞治疗具有良好的耐受性,并在复发性神经淋巴瘤病中显示出有希望的疗效,这是一种难以治疗的疾病,医疗需求未得到满足。 研究结果表明 CD19-CAR 可能充分穿透血液神经屏障。CNS 淋巴瘤的毒性和结局总体上与 CAR-T 细胞疗法相似。
更新日期:2024-10-23
中文翻译:

CD19 定向嵌合抗原受体 T 细胞治疗继发性神经淋巴瘤病后的毒性和结局
周围神经系统 (PNS) 淋巴瘤浸润,称为神经淋巴瘤病,代表一种独特的结外非霍奇金淋巴瘤变体,结果令人沮丧。CD19 定向嵌合抗原受体 (CD19-CAR) T 细胞疗法已成为一种安全有效的 B 细胞淋巴瘤治疗方法。我们旨在评估 CD19-CAR T 细胞在神经淋巴瘤病中的毒性和疗效。在麻省总医院回顾性确定了接受 CD19 CAR T 细胞治疗的神经淋巴瘤病患者,为期 6 年。根据 ASTCT 分类对毒性进行分级,记录管理和反应率。在接受 CD19-CAR T 细胞之前,确定 11 例神经淋巴瘤病患者接受 2 线 PNS 定向治疗的中位数 (范围:1-3)。神经淋巴瘤病局限于神经根 (8/11, 73%)、神经丛 (5/11, 45%)、外周神经 (4/11, 36%) 和颅神经 (5/11, 45%)。在 8/11 (73%;1 级:N = 7;2 级:N = 1) 病例中检测到低级别细胞因子释放综合征 (CRS)。低级别和高级别免疫细胞相关神经毒性综合征 (ICANS) 分别见于 5/11 (45%;1 级:N = 4;2 级:N = 1) 和 1/11 (9%;4 级)患者。输注时 CRP 水平可预测 ICANS (曲线下面积: 0.96,p = 0.01)。11 例神经淋巴瘤病患者中有 7 例 (64%) 对 CD19-CAR T 细胞有反应。3 例 (27%) 达到完全缓解 (CR),其中 2 例患者在输注 CD19-CAR 后 9 个月和 46 个月持续 CR。中位无进展生存期 (PFS) 为 4 个月。总的来说,CD19-CAR T 细胞治疗具有良好的耐受性,并在复发性神经淋巴瘤病中显示出有希望的疗效,这是一种难以治疗的疾病,医疗需求未得到满足。 研究结果表明 CD19-CAR 可能充分穿透血液神经屏障。CNS 淋巴瘤的毒性和结局总体上与 CAR-T 细胞疗法相似。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号