Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of alkene radical cation for thioxanthone-TfOH complex-catalyzed intramolecular cyclization using a photoredox catalysis strategy
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-23 , DOI: 10.1016/j.jcat.2024.115817 Jin Feng, Guanglong Huang, Haoliang Huang, Hanguang Tang, Wangsheng Liu, Aishun Ding, Xiao-Song Xue, Hao Guo
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-23 , DOI: 10.1016/j.jcat.2024.115817 Jin Feng, Guanglong Huang, Haoliang Huang, Hanguang Tang, Wangsheng Liu, Aishun Ding, Xiao-Song Xue, Hao Guo
|
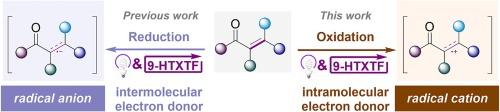
Photo catalysis has comprehensively become a powerful tool in organic synthesis, and organic molecules are thriving as catalyst. The thioxanthone-TfOH complex (9-HTXTF ) as photoredox catalyst with high oxidative capacity can be applied in single electron reduction of alkene affording alkene radical anion as a key intermediate. To transform this intermediate from radical anion to radical cation, a well-designed strategy is proposed with N -arylacrylamides as substrate. Based on its single electron transfer (SET) with 9-HTXTF *, N-radical cation is generated and then transformed to alkene radical cation by intramolecular conjugated system. By using this photoredox catalysis strategy, we developed a 9-HTXTF -catalyzed photochemical cyclization of alkenes, which further expands the applications of this catalyst. The entire cyclization is metal-free and without sacrificing agents, which conforms to atom economy and environmental friendliness.
中文翻译:

使用光氧化还原催化策略生成烯烃自由基阳离子,用于噻杂蒽酮-TfOH 复合物催化的分子内环化
光催化已经全面成为有机合成的有力工具,有机分子作为催化剂蓬勃发展。噻杂蒽酮-TfOH 络合物 (9-HTXTF) 作为具有高氧化容量的光氧化还原催化剂,可用于烯烃的单电子还原,提供烯烃自由基阴离子作为关键中间体。为了将这种中间体从自由基阴离子转化为自由基阳离子,提出了一种以 N-芳基酰胺为底物的良好设计策略。基于其与 9-HTXTF* 的单电子转移 (SET),生成 N-自由基阳离子,然后通过分子内共轭系统转化为烯烃自由基阳离子。通过使用这种光氧化还原催化策略,我们开发了一种 9-HTXTF 催化的烯烃光化学环化反应,进一步扩展了该催化剂的应用。整个环化过程不含金属,无牺牲剂,符合原子经济性和环保性。
更新日期:2024-10-23
中文翻译:

使用光氧化还原催化策略生成烯烃自由基阳离子,用于噻杂蒽酮-TfOH 复合物催化的分子内环化
光催化已经全面成为有机合成的有力工具,有机分子作为催化剂蓬勃发展。噻杂蒽酮-TfOH 络合物 (9-HTXTF) 作为具有高氧化容量的光氧化还原催化剂,可用于烯烃的单电子还原,提供烯烃自由基阴离子作为关键中间体。为了将这种中间体从自由基阴离子转化为自由基阳离子,提出了一种以 N-芳基酰胺为底物的良好设计策略。基于其与 9-HTXTF* 的单电子转移 (SET),生成 N-自由基阳离子,然后通过分子内共轭系统转化为烯烃自由基阳离子。通过使用这种光氧化还原催化策略,我们开发了一种 9-HTXTF 催化的烯烃光化学环化反应,进一步扩展了该催化剂的应用。整个环化过程不含金属,无牺牲剂,符合原子经济性和环保性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号