当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical One-Pot Synthesis of 2-Alkyl-Substituted Benzothiazoles from Bis-(2-nitrophenyl)-disulfides
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-23 , DOI: 10.1021/acs.oprd.4c00136 Volodymyr O. Puskov, Serhii B. Babii, Iryna V. Adamenko, Sviatoslava O. Melnychuk, Tetiana V. Druzhenko, Alexander Yu. Lyapunov, Sergii V. Popov, Dmytro M. Volochnyuk, Serhiy V. Ryabukhin
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-23 , DOI: 10.1021/acs.oprd.4c00136 Volodymyr O. Puskov, Serhii B. Babii, Iryna V. Adamenko, Sviatoslava O. Melnychuk, Tetiana V. Druzhenko, Alexander Yu. Lyapunov, Sergii V. Popov, Dmytro M. Volochnyuk, Serhiy V. Ryabukhin
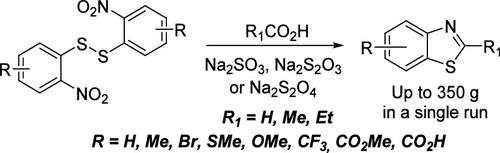
|
2-Methyl benzothiazoles are widely used as key precursors for dyes, photosensitizers, and fluorescent markers. Thus, they are demanded in multigram and even kilogram quantities. We propose a scalable single-step procedure for producing 2-alkyl-substituted benzothiazoles from the corresponding bis-(2-nitrophenyl)-disulfide commercial, technical-grade quality. The reaction scope looks promising; substrates containing various substituents, including carboxylic and ester groups, were introduced. The reaction conditions were carefully optimized according to reducing agents (diverse sodium salts), solvents, ratio, and reaction time. This led to the acquisition of target products in up to 350 g quantities in a single run.
中文翻译:

从双-(2-硝基苯基)-二硫化物中实用的一锅法合成 2-烷基取代的苯并噻唑
2-甲基苯并噻唑被广泛用作染料、光敏剂和荧光标记物的关键前体。因此,它们的需求量为数克甚至公斤。我们提出了一种可扩展的单步程序,用于从相应的双-(2-硝基苯基)-二硫化物商业级质量生产 2-烷基取代的苯并噻唑。反应范围看起来很有希望;引入了包含各种取代基(包括羧基和酯基)的底物。根据还原剂(多种钠盐)、溶剂、比例和反应时间仔细优化反应条件。这导致在一次运行中获得了高达 350 g 的目标产品。
更新日期:2024-10-24
中文翻译:

从双-(2-硝基苯基)-二硫化物中实用的一锅法合成 2-烷基取代的苯并噻唑
2-甲基苯并噻唑被广泛用作染料、光敏剂和荧光标记物的关键前体。因此,它们的需求量为数克甚至公斤。我们提出了一种可扩展的单步程序,用于从相应的双-(2-硝基苯基)-二硫化物商业级质量生产 2-烷基取代的苯并噻唑。反应范围看起来很有希望;引入了包含各种取代基(包括羧基和酯基)的底物。根据还原剂(多种钠盐)、溶剂、比例和反应时间仔细优化反应条件。这导致在一次运行中获得了高达 350 g 的目标产品。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号