当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of N6,3′,5′-Tripivaloyladenosine via Dynamic Kinetic Crystallization and Regioselective Preparation of Pivalated 2′-Deoxy-2′-fluoroarabinoadenosines
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-23 , DOI: 10.1021/acs.oprd.4c00391 Peter E. Maligres, Zhiguo Jake Song, Lu Chen, Birgit Kosjek, Omer Ad, Cheol K. Chung
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-23 , DOI: 10.1021/acs.oprd.4c00391 Peter E. Maligres, Zhiguo Jake Song, Lu Chen, Birgit Kosjek, Omer Ad, Cheol K. Chung
|
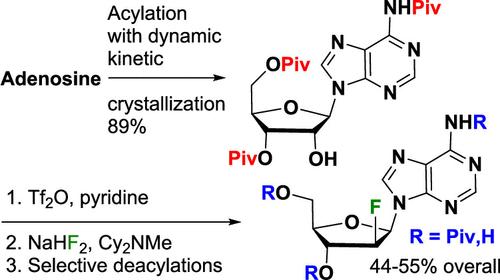
A four chemical step route to 2′-deoxy-2′-fluoro-N6,3′-dipivaloylarabinoadenosine and 2′-deoxy-2′-fluoro-N6-pivaloylarabinoadenosine from adenosine was developed for the preparation of ulevostinag in our STING (Stimulator of Interferon Genes) program. This 4-step route is based on the selective protection of adenosine with a dynamic kinetic crystallization of the desired N6,3′,5′-tripivaloyladenosine. This is followed by activation of the 2′-alcohol as its triflate without pivalate migration. Subsequently, the triflate is displaced with fluoride under mild conditions. Selective deprotection of the esters can give a variety of mono- and diacylated products including the 3′- or 5′-protected 2′-fluoroarabinonucleoside in the presence of the N6-pivalamide.
中文翻译:

通过动态动力学结晶选择性合成 N6,3′,5′-三乙酰腺苷和区域选择性制备 2′-脱氧-2′-氟阿拉伯腺苷
从腺苷中开发出 2'-脱氧-2'-氟-N6,3'-dipivaloylarabinoadenosine 和 2'-脱氧-2'-fluoro-N 6-pivaloylarabinoadenosine 的四化学步骤路线,用于在我们的 STING(干扰素基因刺激剂)计划中制备 ulevostinag。该 4 步路线基于腺苷的选择性保护,具有所需 N6,3′,5′-三硫酰腺苷的动态动力学结晶。随后激活 2′-醇作为其三氟甲磺酸,而没有戊酸酯迁移。随后,在温和的条件下用氟化物置换三氟甲磺酸。酯的选择性脱保护可以得到各种单酰化和二酰化产物,包括在 N6-戊酰胺存在下受 3′ 或 5′ 保护的 2′-氟阿拉伯二核苷。
更新日期:2024-10-23
中文翻译:

通过动态动力学结晶选择性合成 N6,3′,5′-三乙酰腺苷和区域选择性制备 2′-脱氧-2′-氟阿拉伯腺苷
从腺苷中开发出 2'-脱氧-2'-氟-N6,3'-dipivaloylarabinoadenosine 和 2'-脱氧-2'-fluoro-N 6-pivaloylarabinoadenosine 的四化学步骤路线,用于在我们的 STING(干扰素基因刺激剂)计划中制备 ulevostinag。该 4 步路线基于腺苷的选择性保护,具有所需 N6,3′,5′-三硫酰腺苷的动态动力学结晶。随后激活 2′-醇作为其三氟甲磺酸,而没有戊酸酯迁移。随后,在温和的条件下用氟化物置换三氟甲磺酸。酯的选择性脱保护可以得到各种单酰化和二酰化产物,包括在 N6-戊酰胺存在下受 3′ 或 5′ 保护的 2′-氟阿拉伯二核苷。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号