Diabetologia ( IF 8.4 ) Pub Date : 2024-10-23 , DOI: 10.1007/s00125-024-06293-3 Nunzio Guccio, Constanza Alcaino, Emily L. Miedzybrodzka, Marta Santos-Hernández, Christopher A. Smith, Adam Davison, Rula Bany Bakar, Richard G. Kay, Frank Reimann, Fiona M. Gribble
|
Aims/hypothesis
Glucose-dependent insulinotropic polypeptide (GIP) is an incretin hormone secreted by enteroendocrine K cells in the proximal small intestine. This study aimed to explore the function of human K cells at the molecular and cellular levels.
Methods
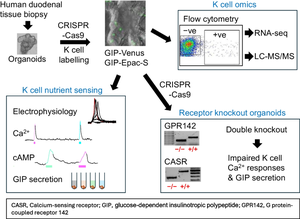
CRISPR-Cas9 homology-directed repair was used to insert transgenes encoding a yellow fluorescent protein (Venus) or an Epac-based cAMP sensor (Epac-S-H187) in the GIP locus in human duodenal-derived organoids. Fluorescently labelled K cells were purified by FACS for RNA-seq and peptidomic analysis. GIP reporter organoids were employed for GIP secretion assays, live-cell imaging of Ca2+ using Fura-2 and cAMP using Epac-S-H187, and basic electrophysiological characterisation. The G protein-coupled receptor genes GPR142 and CASR were knocked out to evaluate roles in amino acid sensing.
Results
RNA-seq of human duodenal K cells revealed enrichment of several G protein-coupled receptors involved in nutrient sensing, including FFAR1, GPBAR1, GPR119, CASR and GPR142. Glucose induced action potential firing and cytosolic Ca2+ elevation and caused a 1.8-fold increase in GIP secretion, which was inhibited by the sodium glucose co-transporter 1/2 (SGLT1/2) blocker sotagliflozin. Activation of the long-chain fatty acid receptor free fatty acid receptor 1 (FFAR1) induced a 2.7-fold increase in GIP secretion, while tryptophan and phenylalanine stimulated secretion by 2.8- and 2.1-fold, respectively. While CASR knockout blunted intracellular Ca2+ responses, a CASR/GPR142 double knockout was needed to reduce GIP secretory responses to aromatic amino acids.
Conclusions/interpretation
The newly generated human organoid K cell model enables transcriptomic and functional characterisation of nutrient-sensing pathways involved in human GIP secretion. Both calcium-sensing receptor (CASR) and G protein-coupled receptor 142 (GPR142) contribute to protein-stimulated GIP secretion. This model will be further used to identify potential targets for modulation of native GIP secretion in diabetes and obesity.
Graphical Abstract
中文翻译:

人十二指肠类器官中葡萄糖依赖性促胰岛素多肽分泌的分子机制
目标/假设
葡萄糖依赖性促胰岛素多肽 (GIP) 是小肠近端肠内分泌 K 细胞分泌的一种肠促胰岛素激素。本研究旨在探索人类 K 细胞在分子和细胞水平上的功能。
方法
CRISPR-Cas9 同源定向修复用于将编码黄色荧光蛋白 (Venus) 或基于 Epac 的 cAMP 传感器 (Epac-S-H187) 的转基因插入人十二指肠衍生类器官的 GIP 基因座中。通过 FACS 纯化荧光标记的 K 细胞,用于 RNA-seq 和肽组学分析。GIP 报告类器官用于 GIP 分泌测定、使用 Fura-2 和 cAMP 使用 Epac-S-H187 进行 Ca2+ 的活细胞成像以及基本的电生理表征。敲除 G 蛋白偶联受体基因 GPR142 和 CASR 以评估在氨基酸感应中的作用。
结果
人十二指肠 K 细胞的 RNA-seq 显示几种参与营养感应的 G 蛋白偶联受体的富集,包括 FFAR1 、 GPBAR1 、 GPR119 、 CASR 和 GPR142。葡萄糖诱导动作电位放电和胞质 Ca2+ 升高,并导致 GIP 分泌增加 1.8 倍,这被钠葡萄糖协同转运蛋白 1/2 (SGLT1/2) 阻滞剂 sotagliflozin 抑制。长链脂肪酸受体游离脂肪酸受体 1 (FFAR1) 的激活诱导 GIP 分泌增加 2.7 倍,而色氨酸和苯丙氨酸刺激分泌分别增加 2.8 倍和 2.1 倍。虽然 CASR 敲除减弱了细胞内 Ca2+ 反应,但需要 CASR/GPR142 双敲除来减少 GIP 分泌对芳香族氨基酸的反应。
结论/解释
新生成的人类类器官 K 细胞模型能够对参与人类 GIP 分泌的营养感应通路进行转录组学和功能表征。钙敏感受体 (CASR) 和 G 蛋白偶联受体 142 (GPR142) 都有助于蛋白质刺激的 GIP 分泌。该模型将进一步用于确定糖尿病和肥胖症中天然 GIP 分泌调节的潜在靶点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号