当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insight into Ni(I)-catalyzed regioselective alkene hydroarylation: is it carbonickelation or hydronickelation?
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-23 , DOI: 10.1039/d4qo01579g Wen-Yan Tong, Zhizheng Chen, Shuanglin Qu, Xiaotai Wang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-23 , DOI: 10.1039/d4qo01579g Wen-Yan Tong, Zhizheng Chen, Shuanglin Qu, Xiaotai Wang
|
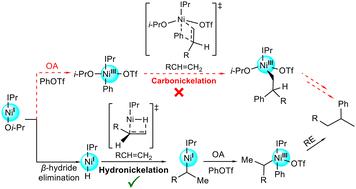
This density functional theory (DFT) study explores the reaction mechanism of nickel-catalyzed hydroarylation of unactivated alkenes that selectively generates branched (Markovnikov) products. Extensive DFT calculations have revealed a novel and plausible mechanism via hydronickelation, which differs from the originally proposed pathway through carbonickelation. The N-heterocyclic carbene-anchored catalyst IPrNiOi-Pr favors β-hydride elimination from the alkoxide ligand over oxidative addition with carbon electrophiles. This chemoselectivity results in the formation of the Ni(I) hydride complex IPrNiH, initiating a hydronickelation pathway involving alkene into Ni–H insertion, PhOTf oxidative addition, and alkyl–phenyl reductive elimination. The β-hydride elimination represents the rate-limiting step, while the oxidative addition of PhOTf determines the regioselectivity, which is sterically controlled. This DFT study demonstrates a robust theoretical–experimental synergy and provides new mechanistic insights valuable for reaction development.
中文翻译:

Ni(I) 催化的区域选择性烯烃加氢芳基化反应的机理见解:是碳镍化还是氢化镍化?
该密度泛函理论 (DFT) 研究探讨了镍催化的未活化烯烃加氢芳基化反应的反应机制,该氢化反应选择性地生成支链 (Markovnikov) 产物。广泛的 DFT 计算揭示了一种通过氢镍化的新颖且合理的机制,这与最初提出的通过碳镍化的途径不同。N-杂环卡宾锚定催化剂 IPrNiOi-Pr 比用亲碳电试剂氧化加成更有利于从醇盐配体中去除β氢化物。这种化学选择性导致 Ni() 氢化物络合物 IPrNiH 的形成,启动了涉及烯烃到 Ni-H 插入、PhOTf 氧化加成和烷基-苯基还原消除的氢镍化途径。β-氢化物消除代表限速步骤,而 PhOTf 的氧化加成决定了区域选择性,这是空间控制的。这项 DFT 研究展示了强大的理论-实验协同作用,并为反应开发提供了有价值的新机理见解。
更新日期:2024-10-23
中文翻译:

Ni(I) 催化的区域选择性烯烃加氢芳基化反应的机理见解:是碳镍化还是氢化镍化?
该密度泛函理论 (DFT) 研究探讨了镍催化的未活化烯烃加氢芳基化反应的反应机制,该氢化反应选择性地生成支链 (Markovnikov) 产物。广泛的 DFT 计算揭示了一种通过氢镍化的新颖且合理的机制,这与最初提出的通过碳镍化的途径不同。N-杂环卡宾锚定催化剂 IPrNiOi-Pr 比用亲碳电试剂氧化加成更有利于从醇盐配体中去除β氢化物。这种化学选择性导致 Ni() 氢化物络合物 IPrNiH 的形成,启动了涉及烯烃到 Ni-H 插入、PhOTf 氧化加成和烷基-苯基还原消除的氢镍化途径。β-氢化物消除代表限速步骤,而 PhOTf 的氧化加成决定了区域选择性,这是空间控制的。这项 DFT 研究展示了强大的理论-实验协同作用,并为反应开发提供了有价值的新机理见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号