Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-22 , DOI: 10.1002/adsc.202401198 Miao Xu, Jia-Tian Jiang, Hao-Xuan Dong, Guang-Hui Wang, Bo Zhou, Long-Wu Ye
|
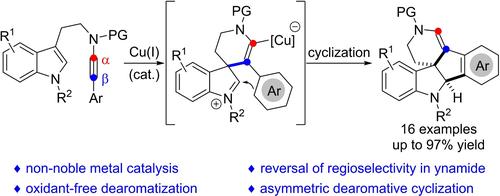
The dearomatization of indoles with ynamides is a convenient access towards polycyclic spiroindolines, which can be initiated by α- and β-additions of ynamides. Currently, the related β-addition initiated dearomative cyclization requires noble-metal catalyst or stoichiometric oxidant. Herein, we report a copper-catalyzed dearomative cyclization of aryl-substituted indolyl ynamides through regioselective β-addition onto ynamides, providing pentacyclic spiroindolines in 81–97% yields with >25:1 diastereoselectivities. Moreover, preliminary success has been obtained for the catalytic enantioselective dearomative cyclization.
中文翻译:

铜催化的吲哚基亚胺脱氧环化反应合成五环螺吲哚啉
用 ynamides 对吲哚的脱芳香化是获得多环螺吲哚啉的便捷途径,这可以通过 ynamides 的 α 和 β 添加来引发。目前,相关的 β 加成引发的脱化学环化需要贵金属催化剂或化学计量氧化剂。在此,我们报道了通过区域选择性β加成到亚胺上芳基取代的吲哚基亚胺的铜催化脱溴环化,以 81-97% 的产率提供五环螺吲哚啉,具有 >25:1 非对映选择性。此外,催化对映选择性脱热环化已取得初步成功。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号