Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure ( IF 4.4 ) Pub Date : 2024-10-22 , DOI: 10.1016/j.str.2024.09.024 William J. Bradshaw, Gemma Harris, Opher Gileadi, Vittorio L. Katis
Structure ( IF 4.4 ) Pub Date : 2024-10-22 , DOI: 10.1016/j.str.2024.09.024 William J. Bradshaw, Gemma Harris, Opher Gileadi, Vittorio L. Katis
|
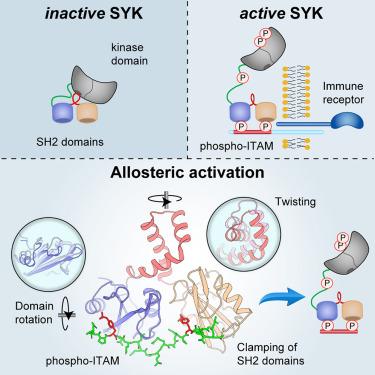
Spleen tyrosine kinase (SYK) is central to adaptive and innate immune signaling. It features a regulatory region containing tandem SH2 (tSH2) domains separated by a helical “hinge” segment keeping SYK inactive by associating with the kinase domain. SYK activation is triggered when the tSH2 domains bind to a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) found on receptor tails. Past mutational studies have indicated that ITAM binding disrupts the hinge-kinase interaction, leading to SYK phosphorylation and activation. However, the mechanism of this process is unclear, as the ITAM interaction occurs far from the hinge region. We have determined crystal structures of three phospho-ITAMs in complex with the tSH2 domains, revealing a highly conserved binding mechanism. These structures, together with mutational studies and biophysical analyses, reveal that phospho-ITAM binding restricts SH2 domain movement and causes allosteric changes in the hinge region. These changes are not compatible with the association of the kinase domain, leading to kinase activation.
中文翻译:

源自多个磷酸化 ITAM 结合结构的 SYK 激酶变构激活机制
脾酪氨酸激酶 (SYK) 是适应性和先天免疫信号传导的核心。它具有一个调节区域,其中包含串联的 SH2 (tSH2) 结构域,该结构域由螺旋“铰链”片段分隔,通过与激酶结构域结合来保持 SYK 失活。当 tSH2 结构域与受体尾部发现的基于磷酸化免疫受体酪氨酸的激活基序 (ITAM) 结合时,会触发 SYK 激活。过去的突变研究表明,ITAM 结合会破坏铰链-激酶相互作用,导致 SYK 磷酸化和激活。然而,这个过程的机制尚不清楚,因为 ITAM 相互作用发生在远离铰链区的地方。我们已经确定了与 tSH2 结构域复合的三种磷酸化 ITAM 的晶体结构,揭示了高度保守的结合机制。这些结构,连同突变研究和生物物理分析,揭示了磷酸化 ITAM 结合限制了 SH2 结构域的运动,并导致铰链区的变构变化。这些变化与激酶结构域的结合不相容,导致激酶激活。
更新日期:2024-10-22
中文翻译:

源自多个磷酸化 ITAM 结合结构的 SYK 激酶变构激活机制
脾酪氨酸激酶 (SYK) 是适应性和先天免疫信号传导的核心。它具有一个调节区域,其中包含串联的 SH2 (tSH2) 结构域,该结构域由螺旋“铰链”片段分隔,通过与激酶结构域结合来保持 SYK 失活。当 tSH2 结构域与受体尾部发现的基于磷酸化免疫受体酪氨酸的激活基序 (ITAM) 结合时,会触发 SYK 激活。过去的突变研究表明,ITAM 结合会破坏铰链-激酶相互作用,导致 SYK 磷酸化和激活。然而,这个过程的机制尚不清楚,因为 ITAM 相互作用发生在远离铰链区的地方。我们已经确定了与 tSH2 结构域复合的三种磷酸化 ITAM 的晶体结构,揭示了高度保守的结合机制。这些结构,连同突变研究和生物物理分析,揭示了磷酸化 ITAM 结合限制了 SH2 结构域的运动,并导致铰链区的变构变化。这些变化与激酶结构域的结合不相容,导致激酶激活。































 京公网安备 11010802027423号
京公网安备 11010802027423号