当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adipocyte-derived glutathione promotes obesity-related breast cancer by regulating the SCARB2-ARF1-mTORC1 complex
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-10-22 , DOI: 10.1016/j.cmet.2024.09.013 Chenxi Zhao, Tingting Zhang, Si-tu Xue, Peitao Zhang, Feng Wang, Yunxuan Li, Ying Liu, Luyao Zhao, Jie Wu, Yechao Yan, Xiaoyun Mao, Yuping Chen, Jian Yuan, Zhuorong Li, Ke Li
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-10-22 , DOI: 10.1016/j.cmet.2024.09.013 Chenxi Zhao, Tingting Zhang, Si-tu Xue, Peitao Zhang, Feng Wang, Yunxuan Li, Ying Liu, Luyao Zhao, Jie Wu, Yechao Yan, Xiaoyun Mao, Yuping Chen, Jian Yuan, Zhuorong Li, Ke Li
|
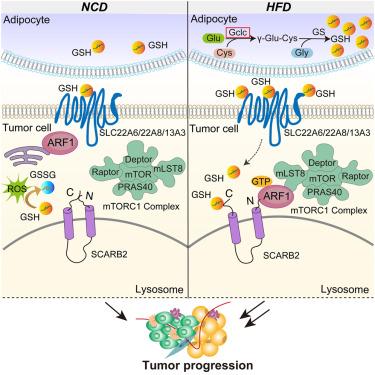
Obesity is a major risk factor for poor breast cancer outcomes, but the impact of obesity-induced tumor microenvironment (TME) metabolites on breast cancer growth and metastasis remains unclear. Here, we performed TME metabolomic analysis in high-fat diet (HFD) mouse models and found that glutathione (GSH) levels were elevated in the TME of obesity-accelerated breast cancer. The deletion of glutamate-cysteine ligase catalytic subunit (GCLC), the rate-limiting enzyme in GSH biosynthesis, in adipocytes but not tumor cells reduced obesity-related tumor progression. Mechanistically, we identified that GSH entered tumor cells and directly bound to lysosomal integral membrane protein-2 (scavenger receptor class B, member 2 [SCARB2]), interfering with the interaction between its N and C termini. This, in turn, recruited mTORC1 to lysosomes through ARF1, leading to the activation of mTOR signaling. Overall, we demonstrated that GSH links obesity and breast cancer progression by acting as an activator of mTOR signaling. Targeting the GSH/SCARB2/mTOR axis could benefit breast cancer patients with obesity.
中文翻译:

脂肪细胞来源的谷胱甘肽通过调节 SCARB2-ARF1-mTORC1 复合物促进肥胖相关乳腺癌
肥胖是乳腺癌预后不良的主要危险因素,但肥胖诱导的肿瘤微环境 (TME) 代谢物对乳腺癌生长和转移的影响仍不清楚。在这里,我们在高脂饮食 (HFD) 小鼠模型中进行了 TME 代谢组学分析,发现谷胱甘肽 (GSH) 水平在肥胖加速乳腺癌的 TME 中升高。脂肪细胞中谷氨酸-半胱氨酸连接酶催化亚基 (GCLC) 的缺失是 GSH 生物合成中的限速酶,在脂肪细胞中而不是肿瘤细胞中,减少了与肥胖相关的肿瘤进展。从机制上讲,我们发现 GSH 进入肿瘤细胞并直接与溶酶体整合膜蛋白-2 (清道夫受体 B 类,成员 2 [SCARB2])结合,干扰其 N 和 C 末端之间的相互作用。反过来,这又通过 ARF1 将 mTORC1 募集到溶酶体,导致 mTOR 信号转导激活。总体而言,我们证明 GSH 通过充当 mTOR 信号转导的激活剂将肥胖与乳腺癌进展联系起来。靶向 GSH/SCARB2/mTOR 轴可能使肥胖乳腺癌患者受益。
更新日期:2024-10-23
中文翻译:

脂肪细胞来源的谷胱甘肽通过调节 SCARB2-ARF1-mTORC1 复合物促进肥胖相关乳腺癌
肥胖是乳腺癌预后不良的主要危险因素,但肥胖诱导的肿瘤微环境 (TME) 代谢物对乳腺癌生长和转移的影响仍不清楚。在这里,我们在高脂饮食 (HFD) 小鼠模型中进行了 TME 代谢组学分析,发现谷胱甘肽 (GSH) 水平在肥胖加速乳腺癌的 TME 中升高。脂肪细胞中谷氨酸-半胱氨酸连接酶催化亚基 (GCLC) 的缺失是 GSH 生物合成中的限速酶,在脂肪细胞中而不是肿瘤细胞中,减少了与肥胖相关的肿瘤进展。从机制上讲,我们发现 GSH 进入肿瘤细胞并直接与溶酶体整合膜蛋白-2 (清道夫受体 B 类,成员 2 [SCARB2])结合,干扰其 N 和 C 末端之间的相互作用。反过来,这又通过 ARF1 将 mTORC1 募集到溶酶体,导致 mTOR 信号转导激活。总体而言,我们证明 GSH 通过充当 mTOR 信号转导的激活剂将肥胖与乳腺癌进展联系起来。靶向 GSH/SCARB2/mTOR 轴可能使肥胖乳腺癌患者受益。






























 京公网安备 11010802027423号
京公网安备 11010802027423号