当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Cyclopropanation of 1,3-Dialkyl Bromides
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-22 , DOI: 10.1021/acs.oprd.4c00302 Sylvain Charvet, Clément Jacob, Aurore Dietsch, Guillaume Tintori, Pierre-Georges Echeverria, Julien C. Vantourout
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-22 , DOI: 10.1021/acs.oprd.4c00302 Sylvain Charvet, Clément Jacob, Aurore Dietsch, Guillaume Tintori, Pierre-Georges Echeverria, Julien C. Vantourout
|
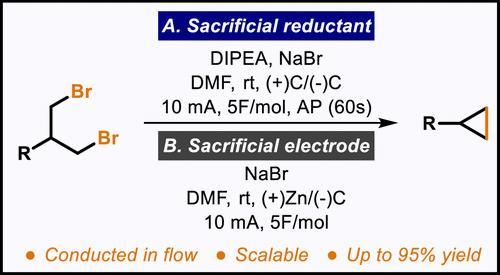
An electrochemical synthesis of mono- and 1,1-disubstituted cyclopropanes is demonstrated. Starting from readily available 1,3-dialkyl bromides, this method hinges on the integration of a sacrificial reductant alongside cost-effective cathode and anode materials. The refined approach eliminates the necessity for a divided cell and the use of hazardous or costly electrodes, thereby streamlining the transition of this protocol to a continuous flow system. In addition, an alternative protocol that utilizes a simple sacrificial anode is also described.
中文翻译:

1,3-二烷基溴化物的电化学环丙烷化
展示了单取代和 1,1-二取代环丙烷的电化学合成。该方法从现成的 1,3-二烷基溴化物开始,取决于牺牲还原剂与具有成本效益的阴极和阳极材料的整合。改进的方法消除了对分体式电池的必要性以及使用危险或昂贵的电极,从而简化了该方案向连续流系统的过渡。此外,还描述了一种利用简单牺牲阳极的替代方案。
更新日期:2024-10-22
中文翻译:

1,3-二烷基溴化物的电化学环丙烷化
展示了单取代和 1,1-二取代环丙烷的电化学合成。该方法从现成的 1,3-二烷基溴化物开始,取决于牺牲还原剂与具有成本效益的阴极和阳极材料的整合。改进的方法消除了对分体式电池的必要性以及使用危险或昂贵的电极,从而简化了该方案向连续流系统的过渡。此外,还描述了一种利用简单牺牲阳极的替代方案。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号