当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent Synthesis of Chiral Hydrobenzofuranpyrrolidines by Catalytic Asymmetric Dearomative Cyclization and Controlled Epimerization
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202401194 Wei Liu, Yeting Huang, Ziqiang Dai, Min Yu, Xuangan Liu, Weijun Yao, Xiaoyu Han
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202401194 Wei Liu, Yeting Huang, Ziqiang Dai, Min Yu, Xuangan Liu, Weijun Yao, Xiaoyu Han
|
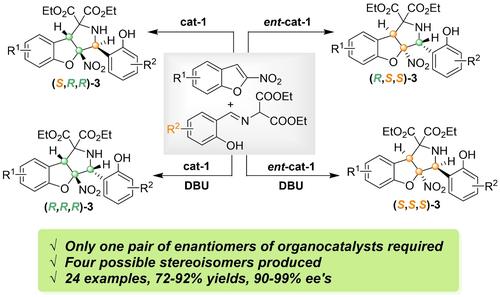
A methodology to access stereoisomeric sets of products bearing multiple stereogenic centers is still a significant challenge in asymmetric catalysis. We present herein our experimental studies on the stereodivergent synthesis of chiral hydrobenzofuran-fused pyrrolidines with three stereogenic centers via organocatalytic asymmetric dearomative cyclization and epimerization process. Chiral bifunctional thiourea catalyst could successfully promote the enantioselective dearomatization cyclization of 2-nitrobenzofurans with o-hydroxy aromatic aldimines, which enabled the synthesis of (3S,3aR,8bR)-hydrobenzofuran[3.2]pyrrolidines in 79–92% yields with >20:1 stereoselectivities and 93–>99% enantio-selectivities. While catalytic amount of DBU could induce the direct intramolecular epimerization of (3S,3aR,8bR)-hydrobenzofuran[3.2] pyrrolidines to its diastereomers (3R,3aR,8bR)-hydrobenzofuran[3.2] pyrrolidines in 72–87% yields without loss of stereoselectivities. The mechanistic pathways of the epimerization process were investigated by a series of control experiments study. This work provides an alternative and forward solution for the stereodivergent preparation of functionalized pyrrolidines with potential bioactivities.
中文翻译:

通过催化不对称脱氧环化和受控差向异构化合成手性氢苯并呋喃吡咯烷
在不对称催化中,获得带有多个立体中心的立体异构产物集的方法仍然是一个重大挑战。我们在此介绍了通过有机催化不对称脱氧环化和差向异构化过程立体合成具有三个立体中心的手性氢苯并呋喃融合吡咯烷的实验研究。手性双功能硫脲催化剂可以成功地促进 2-硝基苯并呋喃与邻羟基芳香族二胺的对映选择性脱芳烃化环化,从而能够以 79-92% 的产率合成 (3S,3aR,8bR)-氢苯并呋喃[3.2]吡咯烷,具有 >20:1 的立体选择性和 93->99% 的对映体选择性。而催化量的 DBU 可以诱导 (3S,3aR,8bR)-氢苯并呋喃[3.2] 吡咯烷直接分子内异构化为其非对映异构体 (3R,3aR,8bR)-氢苯并呋喃[3.2] 吡咯烷,产率为 72-87%,而不会损失立体选择性。通过一系列对照实验研究研究了差向异构化过程的机制途径。这项工作为具有潜在生物活性的功能化吡咯烷的立体发散制备提供了一种替代和正向的解决方案。
更新日期:2024-10-21
中文翻译:

通过催化不对称脱氧环化和受控差向异构化合成手性氢苯并呋喃吡咯烷
在不对称催化中,获得带有多个立体中心的立体异构产物集的方法仍然是一个重大挑战。我们在此介绍了通过有机催化不对称脱氧环化和差向异构化过程立体合成具有三个立体中心的手性氢苯并呋喃融合吡咯烷的实验研究。手性双功能硫脲催化剂可以成功地促进 2-硝基苯并呋喃与邻羟基芳香族二胺的对映选择性脱芳烃化环化,从而能够以 79-92% 的产率合成 (3S,3aR,8bR)-氢苯并呋喃[3.2]吡咯烷,具有 >20:1 的立体选择性和 93->99% 的对映体选择性。而催化量的 DBU 可以诱导 (3S,3aR,8bR)-氢苯并呋喃[3.2] 吡咯烷直接分子内异构化为其非对映异构体 (3R,3aR,8bR)-氢苯并呋喃[3.2] 吡咯烷,产率为 72-87%,而不会损失立体选择性。通过一系列对照实验研究研究了差向异构化过程的机制途径。这项工作为具有潜在生物活性的功能化吡咯烷的立体发散制备提供了一种替代和正向的解决方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号