当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Energetics of PET-Hydrolyzing Enzyme Complexes: A Systematic Comparison from Molecular Dynamics Simulations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-10-21 , DOI: 10.1021/acs.jcim.4c01369 Alessandro Berselli, Maria Cristina Menziani, Francesco Muniz-Miranda
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-10-21 , DOI: 10.1021/acs.jcim.4c01369 Alessandro Berselli, Maria Cristina Menziani, Francesco Muniz-Miranda
|
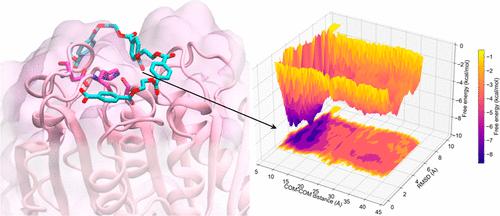
Discovered in 2016, the enzyme PETase, secreted by bacterial Ideonella Sakaiensis 201-F6, has an excellent hydrolytic activity toward poly(ethylene terephthalate) (PET) at room temperature, while it decreases at higher temperatures due to the low thermostability. Many variants have been engineered to overcome this limitation, which hinders industrial application. In this work, we systematically compare PETase wild-type (WT) and four mutants (DuraPETase, ThermoPETase, FastPETase, and HotPETase) using standard molecular dynamics (MD) simulations and unbinding free energy calculations. In particular, we analyze the enzymes’ structural characteristics and binding to a tetrameric PET chain (PET4) under two temperature conditions: T1─300 K and T2─350 K. Our results indicate that (i) PET4 forms stable complexes with the five enzymes at room temperature (∼300 K) and (ii) most of the interactions are localized close to the active site of the protein, where the W185 and Y87 residues interact with the aromatic rings of the substrate. Specifically, (iii) the W185 side-chain explores different conformations in each variant (a phenomenon known in the literature as “W185 wobbling”). This suggests that the binding pocket retains structural plasticity and flexibility among the variants, facilitating substrate recognition and localization events at moderate temperatures. Moreover, (iv) PET4 establishes aromatic interactions with the catalytic H237 residue, stabilizing the catalytic triad composed of residues S160-H237-D206, and helping the system achieve an effective configuration for the hydrolysis reaction. Conversely, (v) the binding affinity decreases at a higher temperature (∼350 K), retaining moderate interactions only for HotPETase. Finally, (vi) MD simulations of complexes formed with poly(ethylene-2,5-furan dicarboxylate) (PEF) show no persistent interactions, suggesting that these enzymes are not yet optimized for binding this alternative semiaromatic plastic polymer. Our study offers valuable insights into the structural stability of these enzymes and the molecular determinants driving PET binding onto their surfaces, sheds light on the mechanistic steps that precede the onset of hydrolysis, and provides a foundation for future enzyme optimization.
中文翻译:

PET 水解酶复合物的结构和能量学:分子动力学模拟的系统比较
2016 年发现的 PETase 酶由细菌 Ideonella Sakaiensis 201-F6 分泌,在室温下对聚对苯二甲酸乙二醇酯 (PET) 具有优异的水解活性,而在高温下由于热稳定性低而降低。许多变体的设计都克服了阻碍工业应用的这一限制。在这项工作中,我们使用标准分子动力学 (MD) 模拟和解结合自由能计算系统地比较了 PETase 野生型 (WT) 和四种突变体 (DuraPETase、ThermoPETase、FastPETase 和 HotPETase)。特别是,我们分析了酶的结构特征以及与四种 PET 链 (PET4) 在两种温度条件下的结合:T1─300 K 和 T2─350 K。我们的结果表明,(i) PET4 在室温 (∼300 K) 下与五种酶形成稳定的复合物,并且 (ii) 大多数相互作用位于蛋白质活性位点附近,其中 W185 和 Y87 残基与底物的芳香环相互作用。具体来说,(iii) W185 侧链探索了每种变体的不同构象(这种现象在文献中称为“W185 摆动”)。这表明结合口袋在变体之间保持结构可塑性和柔韧性,从而促进中等温度下的底物识别和定位事件。此外,(iv) PET4 与催化 H237 残基建立芳香族相互作用,稳定由残基 S160-H237-D206 组成的催化三联体,并帮助系统实现水解反应的有效构型。相反,(v) 结合亲和力在较高温度 (∼350 K) 下降低,仅保留 HotPETase 的适度相互作用。 最后,(vi) 与聚(乙烯-2,5-呋喃二羧酸酯)(PEF) 形成的复合物的 MD 模拟显示没有持续的相互作用,这表明这些酶尚未针对结合这种替代的半芳香族塑料聚合物进行优化。我们的研究为这些酶的结构稳定性以及驱动 PET 结合到其表面的分子决定因素提供了有价值的见解,阐明了水解开始之前的机制步骤,并为未来的酶优化提供了基础。
更新日期:2024-10-22
中文翻译:

PET 水解酶复合物的结构和能量学:分子动力学模拟的系统比较
2016 年发现的 PETase 酶由细菌 Ideonella Sakaiensis 201-F6 分泌,在室温下对聚对苯二甲酸乙二醇酯 (PET) 具有优异的水解活性,而在高温下由于热稳定性低而降低。许多变体的设计都克服了阻碍工业应用的这一限制。在这项工作中,我们使用标准分子动力学 (MD) 模拟和解结合自由能计算系统地比较了 PETase 野生型 (WT) 和四种突变体 (DuraPETase、ThermoPETase、FastPETase 和 HotPETase)。特别是,我们分析了酶的结构特征以及与四种 PET 链 (PET4) 在两种温度条件下的结合:T1─300 K 和 T2─350 K。我们的结果表明,(i) PET4 在室温 (∼300 K) 下与五种酶形成稳定的复合物,并且 (ii) 大多数相互作用位于蛋白质活性位点附近,其中 W185 和 Y87 残基与底物的芳香环相互作用。具体来说,(iii) W185 侧链探索了每种变体的不同构象(这种现象在文献中称为“W185 摆动”)。这表明结合口袋在变体之间保持结构可塑性和柔韧性,从而促进中等温度下的底物识别和定位事件。此外,(iv) PET4 与催化 H237 残基建立芳香族相互作用,稳定由残基 S160-H237-D206 组成的催化三联体,并帮助系统实现水解反应的有效构型。相反,(v) 结合亲和力在较高温度 (∼350 K) 下降低,仅保留 HotPETase 的适度相互作用。 最后,(vi) 与聚(乙烯-2,5-呋喃二羧酸酯)(PEF) 形成的复合物的 MD 模拟显示没有持续的相互作用,这表明这些酶尚未针对结合这种替代的半芳香族塑料聚合物进行优化。我们的研究为这些酶的结构稳定性以及驱动 PET 结合到其表面的分子决定因素提供了有价值的见解,阐明了水解开始之前的机制步骤,并为未来的酶优化提供了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号