Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202400907 Ramachandra Reddy Putta, Junhwa Hong, Seung Hyun Choi, Jinwoo Lee, Honghui Lee, Seok Beom Lee, Suckchang Hong
|
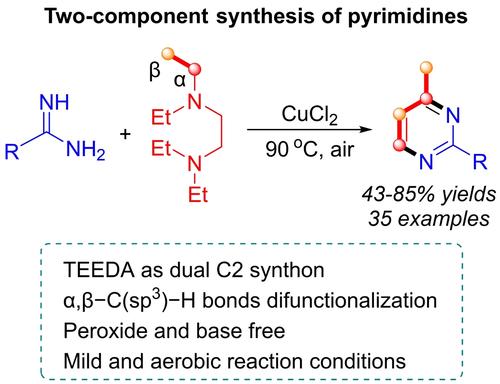
Herein, we describe a two-component methodology developed for the synthesis of pyrimidine derivatives using tertiary alkylamines and amidines. Tertiary alkylamines serve as dual C2 synthons through copper-catalyzed aerobic difunctionalization of the α,β−C(sp3)−H bonds. The process operates under mild conditions and uses atmospheric oxygen as the oxidant. Notably, by this methodology yields up to 85% yields are obtained and a broad substrate scope is shown. Mechanistic studies indicate that the annulation proceeds via a radical-mediated oxidation and C−N bond coupling process. This approach provides a pathway for synthesizing various heterocycles by employing tertiary alkylamines as dual C2 synthons.
中文翻译:

合成嘧啶的双 C2 synthon 策略:铜催化的叔烷基胺的需氧 α,β-C(sp3)−H 键双官能化
在本文中,我们描述了一种为使用叔烷基胺和脒合成嘧啶衍生物而开发的双组分方法。叔烷基胺通过铜催化的 α,β-C(sp3)-H 键的有氧双官能化用作双 C2 合成子。该工艺在温和的条件下运行,并使用大气中的氧气作为氧化剂。值得注意的是,通过这种方法,可获得高达 85% 的产量,并显示出广泛的底物范围。机理研究表明,环化是通过自由基介导的氧化和 C-N 键耦合过程进行的。这种方法通过使用叔烷基胺作为双 C2 合成子,为合成各种杂环提供了一种途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号