Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202401118 Zhixi Zhu, Yue Ma, Xiaoying Xie, Zhiyuan Wang, Lu Han, Jingwu Song, Yuzong Liang, Gerrit J. Poelarends, Yijun Chen, Meiling Lu, Jielin Zhang
|
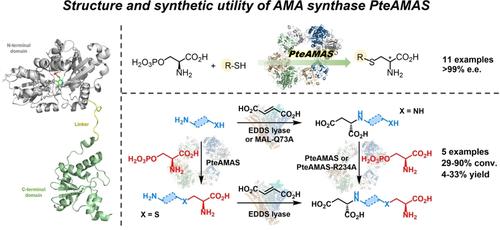
The fungal natural product aspergillomarasmine A (AMA) is an inhibitor of metallo-β-lactamases and a promising co-drug candidate for reversing bacterial β-lactam resistance. The biosynthesis of AMA from L-Asp and O-phospho-L-serine is catalyzed by AMA synthase, which possesses high synthetic potential for the biocatalytic preparation of AMA and related aminopolycarboxylic acids. Here, we identified and structurally characterized an AMA synthase from Pyrenophora teres f. teres 0–1 (PteAMAS). Crystallographic analysis revealed a unique homotetrameric fold and a highly hydrophilic active site, which supports the PLP-dependent β-replacement catalytic mechanism. Furthermore, we demonstrated the utility of PteAMAS for the biocatalytic synthesis of AMA and its analogues, achieving structural diversification of the APA1 moiety through a modular two-enzyme system involving PteAMAS and a selective C−N lyase, namely EDDS lyase or 3-methylaspartate ammonia lyase variant MAL−Q73 A. In addition, we exhibited the usefulness of PteAMAS for convenient synthesis of various S-alkyl, -aryl, and -arylalkyl substituted L-cysteines, which are valuable non-canonical amino acids with broad pharmaceutical applications. The present study has not only provided the structural basis for catalysis and substrate recognition by PteAMAS, but also paved the way for biocatalytic preparation of structurally complex AMA analogues and various non-canonical amino acids.
中文翻译:

来自 Pyrenophora teres f. teres 0-1 的 AMA 合酶用于合成曲霉菌胺 A 类似物和非经典氨基酸的研究
真菌天然产物曲霉马拉辛 A (AMA) 是金属β-内酰胺酶的抑制剂,是逆转细菌 β-内酰胺耐药性的有前途的联合候选药物。AMA 合酶催化 LAsp 和 O-磷酸-L-丝氨酸生物合成 AMA,其具有很高的合成潜力,可用于生物催化制备 AMA 和相关氨基聚羧酸。在这里,我们鉴定并结构表征了来自 Pyrenophora teres f. teres 0-1 (PteAMAS) 的 AMA 合酶。晶体学分析揭示了独特的同源四聚体折叠和高度亲水性活性位点,这支持 PLP 依赖性 β 取代催化机制。此外,我们证明了 PteAMAS 用于 AMA 及其类似物的生物催化合成的效用,通过涉及 PteAMAS 和选择性 C-N 裂解酶的模块化双酶系统实现 APA1 部分的结构多样化,即 EDDS 裂解酶或 3-甲基天冬氨酸裂氨酶变体 MAL-Q73 A。此外,我们还展示了 PteAMAS 在方便合成各种 S-烷基、-芳基和 -芳基烷基取代的 L-半胱氨酸方面的有用性,这些氨基酸是具有广泛药学应用的有价值的非经典氨基酸。本研究不仅为 PteAMAS 的催化和底物识别提供了结构基础,而且为生物催化制备结构复杂的 AMA 类似物和各种非经典氨基酸铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号