当前位置:
X-MOL 学术
›
J. Mater. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-functions of the carbon-confined oxygen on the capacitance and cycle stability enhancements of Zn-ion capacitors
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-10-21 , DOI: 10.1016/j.jmst.2024.10.003 Yi Zhang, Zhimin Zou, Qi Liu, Yu Qiao, Chunhai Jiang
Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2024-10-21 , DOI: 10.1016/j.jmst.2024.10.003 Yi Zhang, Zhimin Zou, Qi Liu, Yu Qiao, Chunhai Jiang
|
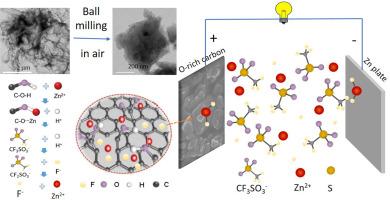
Zinc-ion capacitors (ZICs) are promising energy storage devices due to their balance between the energy and power densities inherited from Zn-ion batteries and supercapacitors, respectively. However, the low specific capacitance of carbon cathode materials and the dendrite growth on Zn anode have set fatal drawbacks to their energy density and cycle stability. Herein, we demonstrate that, in 1 M Zn(CF3 SO3 )2 /DMF (N, N-dimethylformamide) electrolyte, confining oxygen in carbon cathode materials via high-energy ball milling can synergistically introduce additional pseudocapacitance on the cathode side while suppressing the dendrite growth on Zn anode side, which jointly lead to high energy density (94 Wh kg−1 at 448 W kg−1 ) and long cycle stability of ZICs. The hydroxyl group in carbon cathode can be transformed to C–O–Zn together with the release of protons during the initial discharge, which in turn stimulates the defluorination of C F 3 SO 3 − 2 on both cathode and anode. The ZnF2 formed on the surface of the Zn anode suppresses the dendrite growth by regulating the Zn2+ deposition/stripping in a reticular structure, resulting in the excellent cycle stability. This work provides a facile strategy to rationally design and construct high energy and stable ZICs through engineering the oxygen-bearing functional groups in carbon cathode materials.
中文翻译:

碳限制氧对 Zn 离子电容器电容和周期稳定性的双重功能增强
锌离子电容器 (ZIC) 是很有前途的储能设备,因为它们分别继承了锂离子电池和超级电容器的能量和功率密度之间的平衡。然而,碳正极材料的低比电容和 Zn 负极上的枝晶生长对其能量密度和循环稳定性造成了致命的不利影响。在此,我们证明,在 1 M Zn(CF3SO3)2/DMF (N, N-dimethylformamide) 电解质中,通过高能球磨将氧限制在碳正极材料中可以协同在阴极侧引入额外的伪电容,同时抑制 Zn 负极侧的枝晶生长,共同导致 ZIC 的高能量密度(448 W kg-1 时为 94 Wh kg-1)和长循环稳定性。碳阴极中的羟基可以在初始放电过程中随着质子的释放一起转化为 C-O-Zn,这反过来又刺激 CF3SO3− 阴离子的脱氟和形成 ZnF2 在阴极和阳极上。ZnF2 形成在 Zn 阳极表面,通过调节 Zn2+ 网状结构中的沉积/剥离来抑制枝晶生长,从而获得优异的循环稳定性。这项工作提供了一种简单的策略,通过对碳正极材料中的含氧官能团进行工程改造,合理地设计和构建高能量和稳定的 ZIC。
更新日期:2024-10-21
中文翻译:

碳限制氧对 Zn 离子电容器电容和周期稳定性的双重功能增强
锌离子电容器 (ZIC) 是很有前途的储能设备,因为它们分别继承了锂离子电池和超级电容器的能量和功率密度之间的平衡。然而,碳正极材料的低比电容和 Zn 负极上的枝晶生长对其能量密度和循环稳定性造成了致命的不利影响。在此,我们证明,在 1 M Zn(CF3SO3)2/DMF (N, N-dimethylformamide) 电解质中,通过高能球磨将氧限制在碳正极材料中可以协同在阴极侧引入额外的伪电容,同时抑制 Zn 负极侧的枝晶生长,共同导致 ZIC 的高能量密度(448 W kg-1 时为 94 Wh kg-1)和长循环稳定性。碳阴极中的羟基可以在初始放电过程中随着质子的释放一起转化为 C-O-Zn,这反过来又刺激 CF3SO3− 阴离子的脱氟和形成 ZnF2 在阴极和阳极上。ZnF2 形成在 Zn 阳极表面,通过调节 Zn2+ 网状结构中的沉积/剥离来抑制枝晶生长,从而获得优异的循环稳定性。这项工作提供了一种简单的策略,通过对碳正极材料中的含氧官能团进行工程改造,合理地设计和构建高能量和稳定的 ZIC。

































 京公网安备 11010802027423号
京公网安备 11010802027423号