当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating Molecular Interactions in Bulk and Electrochemical Interfaces of Deep Eutectic Solvent-Based Tailored Electrolytes for Facilitating Reactive CO2 Capture
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-10-21 , DOI: 10.1021/acssuschemeng.4c05394 Cini M. Suresh, Mrityunjay K. Jha, Navneet, Hemant K. Kashyap, Pravin Popinand Ingole
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-10-21 , DOI: 10.1021/acssuschemeng.4c05394 Cini M. Suresh, Mrityunjay K. Jha, Navneet, Hemant K. Kashyap, Pravin Popinand Ingole
|
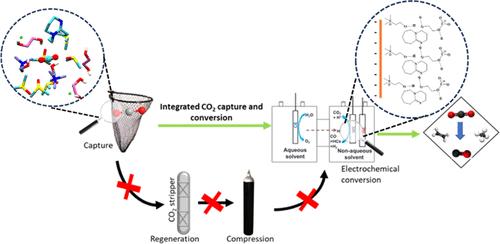
The conventional CO2 capture and utilization (CCU) uses aqueous amine solutions, but its environmental hostility and energy-intensive process to regenerate CO2 are major hurdles toward sustainability. Alternatively, electrochemical reactive CO2 capture (eRCC) that integrates CO2 capture and its conversion has been considered a promising method for economical and sustainable CO2 valorization. However, designing a suitable electrolyte system with tailored electrochemical interfaces for efficient eRCC is a major challenge. Herein, we report a tailored deep eutectic solvent (DES)-based electrolyte containing a superbase (DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene), an aprotic diluent (DMSO: dimethyl sulfoxide), and ethaline (Eth) for an efficient and low-cost eRCC. The tailored DES electrolyte depicts multifold improvement in eRCC performance compared to pristine Eth with good CO2 capture capacity and a superior conversion rate (363.6 μmol cm–2 h–1) at elevated temperatures (i.e., 50 °C). The spectroscopic, electrochemical, and theoretical (AIMD) investigations suggest that the modulated molecular interactions between DES and CO2 boost its capture and facilitate the release of captured CO2 for subsequent reduction. Overall, the facile mass transport, higher concentration of CO2 at the electrode surface, and greater stabilization of intermediates due to the formation of a compact electrical double layer in a tailored DES resulted in relatively high eRCC performance.
中文翻译:

调节基于深共晶溶剂的定制电解质的体界面和电化学界面中的分子相互作用,以促进反应性 CO2 捕获
传统的 CO2 捕获和利用 (CCU) 使用胺水溶液,但其环境敌意和能源密集型的 CO2 再生过程是实现可持续发展的主要障碍。或者,将 CO2 捕获及其转化集成的电化学反应性 CO2 捕获 (eRCC) 被认为是一种经济且可持续的 CO2 增值的有前途的方法。然而,为高效的 eRCC 设计具有定制电化学界面的合适电解质系统是一项重大挑战。在此,我们报道了一种定制的基于低共熔溶剂 (DES) 的电解质,其中包含超碱(DBU:1,8-二氮杂双环[5.4.0]undec-7-ene)、非质子稀释剂(DMSO:二甲基亚砜)和乙啉 (Eth),用于高效和低成本的 eRCC。与原始 Eth 相比,定制的 DES 电解质在高温(即 50 °C)下具有良好的 CO2 捕获能力和卓越的转化率 (363.6 μmol cm–2 h–1),因此定制的 DES 电解质的 eRCC 性能提高了数倍。光谱、电化学和理论 (AIMD) 研究表明,DES 和 CO2 之间的调节分子相互作用促进了其捕获并促进捕获的 CO2 的释放,以便随后进行还原。总体而言,由于在定制的 DES 中形成致密的双电层,简单的质量传递、电极表面的较高浓度 CO2 以及由于在定制的 DES 中形成致密的双电层而导致的中间体的更大稳定性,导致了相对较高的 eRCC 性能。
更新日期:2024-10-21
中文翻译:

调节基于深共晶溶剂的定制电解质的体界面和电化学界面中的分子相互作用,以促进反应性 CO2 捕获
传统的 CO2 捕获和利用 (CCU) 使用胺水溶液,但其环境敌意和能源密集型的 CO2 再生过程是实现可持续发展的主要障碍。或者,将 CO2 捕获及其转化集成的电化学反应性 CO2 捕获 (eRCC) 被认为是一种经济且可持续的 CO2 增值的有前途的方法。然而,为高效的 eRCC 设计具有定制电化学界面的合适电解质系统是一项重大挑战。在此,我们报道了一种定制的基于低共熔溶剂 (DES) 的电解质,其中包含超碱(DBU:1,8-二氮杂双环[5.4.0]undec-7-ene)、非质子稀释剂(DMSO:二甲基亚砜)和乙啉 (Eth),用于高效和低成本的 eRCC。与原始 Eth 相比,定制的 DES 电解质在高温(即 50 °C)下具有良好的 CO2 捕获能力和卓越的转化率 (363.6 μmol cm–2 h–1),因此定制的 DES 电解质的 eRCC 性能提高了数倍。光谱、电化学和理论 (AIMD) 研究表明,DES 和 CO2 之间的调节分子相互作用促进了其捕获并促进捕获的 CO2 的释放,以便随后进行还原。总体而言,由于在定制的 DES 中形成致密的双电层,简单的质量传递、电极表面的较高浓度 CO2 以及由于在定制的 DES 中形成致密的双电层而导致的中间体的更大稳定性,导致了相对较高的 eRCC 性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号