当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multi-step chirality transfer and racemization kinetics of pillar [5]arenes by tuning the halogen substituents on the rims
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-22 , DOI: 10.1039/d4qo01799d Lizhi Fang, Xiaowen Guan, Yanling Shen, Dayang Zhou, Long Chen, Xiaochuan Chen, Wanhua Wu, Leyong Wang, Cheng Yang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-22 , DOI: 10.1039/d4qo01799d Lizhi Fang, Xiaowen Guan, Yanling Shen, Dayang Zhou, Long Chen, Xiaochuan Chen, Wanhua Wu, Leyong Wang, Cheng Yang
|
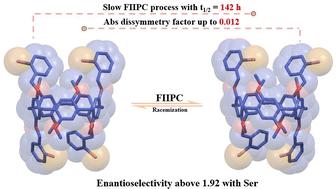
Halogen-substituted pillar[5]arenes exhibited hindered hydroquinone subunit flipping, enabling enantioseparation. An enantiopure conformer showed a dissymmetric factor of 0.012 and an enantioselective binding ratio exceeding 1.92. Complexation with L-serine induced an enantiopreference in pillar[5]arene, which persisted for a certain period after chiral inducer removal and reversed upon the addition of D-serine. This induced enantiopreference was subsequently transferred to a chromophore guest, achieving a multi-step chiral transfer process. This approach offers a potential alternative to traditional chiral HPLC methods, enabling the manipulation of chiral recognition and transfer through chiral inducers.
中文翻译:

通过调整轮辋上的卤素取代基,实现柱 [5] 芳烃的多步手性转移和外消旋化动力学
卤素取代的柱[5]芳烃表现出受阻的对苯二酚亚基翻转,使对映体分离成为可能。对映体纯构象异构体显示不对称因子为 0.012,对映选择性结合比超过 1.92。与 L-丝氨酸的络合诱导柱 [5] 芳烃中的对映体偏好,该对抗体在手性诱导剂去除后持续一段时间,并在加入 D-丝氨酸时逆转。这种诱导的对映异性随后被转移到发色团客体上,实现了多步手性转移过程。这种方法为传统的手性 HPLC 方法提供了一种潜在的替代方案,能够通过手性诱导剂操纵手性识别和转移。
更新日期:2024-10-22
中文翻译:

通过调整轮辋上的卤素取代基,实现柱 [5] 芳烃的多步手性转移和外消旋化动力学
卤素取代的柱[5]芳烃表现出受阻的对苯二酚亚基翻转,使对映体分离成为可能。对映体纯构象异构体显示不对称因子为 0.012,对映选择性结合比超过 1.92。与 L-丝氨酸的络合诱导柱 [5] 芳烃中的对映体偏好,该对抗体在手性诱导剂去除后持续一段时间,并在加入 D-丝氨酸时逆转。这种诱导的对映异性随后被转移到发色团客体上,实现了多步手性转移过程。这种方法为传统的手性 HPLC 方法提供了一种潜在的替代方案,能够通过手性诱导剂操纵手性识别和转移。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号