Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zonal patterning of extracellular matrix and stromal cell populations along a perfusable cellular microchannel
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-10-21 , DOI: 10.1039/d4lc00579a Brea Chernokal, Bryan J. Ferrick, Jason P. Gleghorn
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-10-21 , DOI: 10.1039/d4lc00579a Brea Chernokal, Bryan J. Ferrick, Jason P. Gleghorn
|
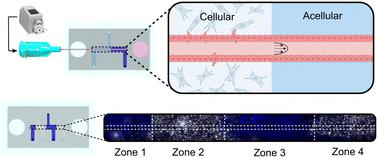
The spatial organization of biophysical and biochemical cues in the extracellular matrix (ECM) in concert with reciprocal cell–cell signaling is vital to tissue patterning during development. However, elucidating the role an individual microenvironmental factor plays using existing in vivo models is difficult due to their inherent complexity. In this work, we have developed a microphysiological system to spatially pattern the biochemical, biophysical, and stromal cell composition of the ECM along an epithelialized 3D microchannel. This technique is adaptable to multiple hydrogel compositions and scalable to the number of zones patterned. We confirmed that the methodology to create distinct zones resulted in a continuous, annealed hydrogel with regional interfaces that did not hinder the transport of soluble molecules. Further, the interface between hydrogel regions did not disrupt microchannel structure, epithelial lumen formation, or media perfusion through an acellular or cellularized microchannel. Finally, we demonstrated spatially patterned tubulogenic sprouting of a continuous epithelial tube into the surrounding hydrogel confined to local regions with stromal cell populations, illustrating spatial control of cell–cell interactions and signaling gradients. This easy-to-use system has wide utility for modeling three-dimensional epithelial and endothelial tissue interactions with heterogeneous hydrogel compositions and/or stromal cell populations to investigate their mechanistic roles during development, homeostasis, or disease.
中文翻译:

细胞外基质和基质细胞群沿可灌注细胞微通道的分区模式
细胞外基质 (ECM) 中生物物理和生化线索的空间组织与相互的细胞间信号传导相一致,对于发育过程中的组织模式至关重要。然而,由于其固有的复杂性,使用现有的体内模型来阐明单个微环境因素所起的作用是困难的。在这项工作中,我们开发了一种微生理系统,用于沿上皮化 3D 微通道在空间上模式 ECM 的生化、生物物理和基质细胞组成。该技术适用于多种水凝胶组合物,并可根据图案化的区域数量进行扩展。我们证实,创建不同区域的方法导致了连续的退火水凝胶,其区域界面不会阻碍可溶性分子的运输。此外,水凝胶区域之间的界面不会破坏微通道结构、上皮管腔形成或通过脱细胞或细胞化微通道的培养基灌注。最后,我们证明了连续上皮管的空间模式化管状发芽到局限于基质细胞群局部区域的周围水凝胶中,说明了细胞间相互作用和信号梯度的空间控制。这种易于使用的系统具有广泛的用途,可用于模拟三维上皮和内皮组织与异质水凝胶组成和/或基质细胞群的相互作用,以研究它们在发育、稳态或疾病过程中的机制作用。
更新日期:2024-10-21
中文翻译:

细胞外基质和基质细胞群沿可灌注细胞微通道的分区模式
细胞外基质 (ECM) 中生物物理和生化线索的空间组织与相互的细胞间信号传导相一致,对于发育过程中的组织模式至关重要。然而,由于其固有的复杂性,使用现有的体内模型来阐明单个微环境因素所起的作用是困难的。在这项工作中,我们开发了一种微生理系统,用于沿上皮化 3D 微通道在空间上模式 ECM 的生化、生物物理和基质细胞组成。该技术适用于多种水凝胶组合物,并可根据图案化的区域数量进行扩展。我们证实,创建不同区域的方法导致了连续的退火水凝胶,其区域界面不会阻碍可溶性分子的运输。此外,水凝胶区域之间的界面不会破坏微通道结构、上皮管腔形成或通过脱细胞或细胞化微通道的培养基灌注。最后,我们证明了连续上皮管的空间模式化管状发芽到局限于基质细胞群局部区域的周围水凝胶中,说明了细胞间相互作用和信号梯度的空间控制。这种易于使用的系统具有广泛的用途,可用于模拟三维上皮和内皮组织与异质水凝胶组成和/或基质细胞群的相互作用,以研究它们在发育、稳态或疾病过程中的机制作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号