当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Reductive Coupling of Nitro Compounds with Grignard and Organozinc Reagents for Synthesis of Functionalized Secondary Amines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202401034 Takeshi Hata, Koki Nishi, Daiki Goto, Yuta Tatsumi, Shoma Kobayashi, Masayuki Shigeta, Hirokazu Urabe
中文翻译:

硝基化合物与格氏试剂和有机锌试剂的铁催化还原偶联反应合成官能化仲胺
更新日期:2024-10-21
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202401034 Takeshi Hata, Koki Nishi, Daiki Goto, Yuta Tatsumi, Shoma Kobayashi, Masayuki Shigeta, Hirokazu Urabe
|
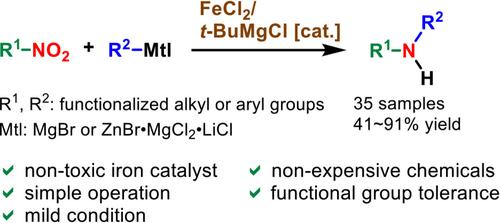
The treatment of nitro compounds with Grignard reagents in the presence of a catalytic amount of FeCl2/t-BuMgCl produced various types of secondary amines in good yields. This reductive coupling most likely involves the compatibility of functional groups such as chlorine, benzyl ether, olefin, and acetylene. Moreover, the corresponding poly-substituted amines were not observed under these conditions. When the Grignard reagents were replaced with organozinc reagents, functionalized secondary amines containing ester and nitrile groups were obtained in good yields.
中文翻译:

硝基化合物与格氏试剂和有机锌试剂的铁催化还原偶联反应合成官能化仲胺
在催化量的 FeCl2/t-BuMgCl 存在下,用 Grignard 试剂处理硝基化合物,以良好的收率产生各种类型的仲胺。这种还原性偶联很可能涉及氯、苄醚、烯烃和乙炔等官能团的相容性。此外,在这些条件下未观察到相应的多取代胺。当用有机锌试剂代替 Grignard 试剂时,以良好的产率获得含有酯和腈基的官能化仲胺。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号