当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arylation of Secondary Phosphines with Diaryliodonium Salts under Metal-Free and Non-Photochemical Conditions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202400919 Ajit Kumar Jha, Sudeep Sarkar, Kacper Szczepanski, Marcin Kalek
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-10-21 , DOI: 10.1002/adsc.202400919 Ajit Kumar Jha, Sudeep Sarkar, Kacper Szczepanski, Marcin Kalek
|
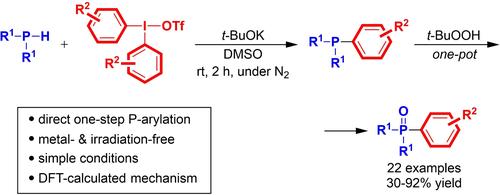
A metal- and irradiation-free approach for the direct arylation of secondary phosphines has been developed. The reaction employs diaryliodonium salts as electrophilic aryl-transfer reagents, effecting the P−Ar bond-formation in a single step, under mild conditions, using t-BuOK as a base and DMSO as a solvent. The protocol furnishes expedient access to diverse unsymmetrical triarylphosphines and, upon a one-pot oxidation, the corresponding triarylphosphine oxides. Experimental and computational studies support the inner sphere aryl-transfer mechanism through a reductive coupling at the hypervalent iodine center. The calculations also point to a key role played by potassium ions, providing extra stabilization to the P−C bond-forming transition state by binding the reactants via cation-π interactions.
中文翻译:

在无金属和非光化学条件下,仲膦与二芳基二烯铵盐的芳基化反应
已经开发了一种用于次级膦直接芳基化的无金属和无辐照方法。该反应采用二芳基盐作为亲芳基转移试剂,在温和条件下,使用 t-BuOK 作为碱,以 DMSO 为溶剂,一步实现 P-Ar 键的形成。该方案提供了对各种不对称的三芳基膦的便利访问,并且在一锅法氧化后,提供了相应的三芳基膦氧化物。实验和计算研究通过高价碘中心的还原耦合支持内球芳基转移机制。计算还指出了钾离子发挥的关键作用,它通过阳离子-π相互作用结合反应物,为 P-C 键形成过渡态提供额外的稳定性。
更新日期:2024-10-21
中文翻译:

在无金属和非光化学条件下,仲膦与二芳基二烯铵盐的芳基化反应
已经开发了一种用于次级膦直接芳基化的无金属和无辐照方法。该反应采用二芳基盐作为亲芳基转移试剂,在温和条件下,使用 t-BuOK 作为碱,以 DMSO 为溶剂,一步实现 P-Ar 键的形成。该方案提供了对各种不对称的三芳基膦的便利访问,并且在一锅法氧化后,提供了相应的三芳基膦氧化物。实验和计算研究通过高价碘中心的还原耦合支持内球芳基转移机制。计算还指出了钾离子发挥的关键作用,它通过阳离子-π相互作用结合反应物,为 P-C 键形成过渡态提供额外的稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号