当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
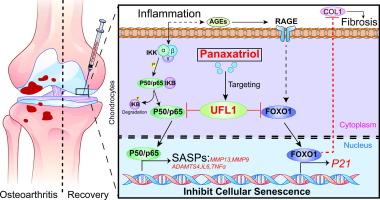
Panaxatriol exerts anti-senescence effects and alleviates osteoarthritis and cartilage repair fibrosis by targeting UFL1
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-21 , DOI: 10.1016/j.jare.2024.10.016 Biao Kuang, Nana Geng, Miao Yi, Qiqi Zeng, Mengtian Fan, Menglin Xian, Lin Deng, Cheng Chen, Yiming Pan, Liang Kuang, Fengtao Luo, Yangli Xie, Chao Liu, Zhongliang Deng, Mao Nie, Yu Du, Fengjin Guo
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-10-21 , DOI: 10.1016/j.jare.2024.10.016 Biao Kuang, Nana Geng, Miao Yi, Qiqi Zeng, Mengtian Fan, Menglin Xian, Lin Deng, Cheng Chen, Yiming Pan, Liang Kuang, Fengtao Luo, Yangli Xie, Chao Liu, Zhongliang Deng, Mao Nie, Yu Du, Fengjin Guo
|
Osteoarthritis (OA), the most common degenerative joint disease, can eventually lead to disability. However, no safe or effective intervention is currently available. Therefore, there is an urgent need to develop effective drugs that reduce cartilage damage and treat OA.
中文翻译:

Panaxatriol 通过靶向 UFL1 发挥抗衰老作用并缓解骨关节炎和软骨修复纤维化
骨关节炎 (OA) 是最常见的退行性关节疾病,最终会导致残疾。然而,目前尚无安全有效的干预措施。因此,迫切需要开发减少软骨损伤和治疗 OA 的有效药物。
更新日期:2024-10-21
中文翻译:

Panaxatriol 通过靶向 UFL1 发挥抗衰老作用并缓解骨关节炎和软骨修复纤维化
骨关节炎 (OA) 是最常见的退行性关节疾病,最终会导致残疾。然而,目前尚无安全有效的干预措施。因此,迫切需要开发减少软骨损伤和治疗 OA 的有效药物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号