当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective syn-1,2-hydroarylation of internal alkynes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-19 , DOI: 10.1039/d4qo01715c Shubham Dutta, Manoj Sethi, Avijit Maity, Aradhana Sahoo, Vincent Gandon, Akhila K. Sahoo
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-10-19 , DOI: 10.1039/d4qo01715c Shubham Dutta, Manoj Sethi, Avijit Maity, Aradhana Sahoo, Vincent Gandon, Akhila K. Sahoo
|
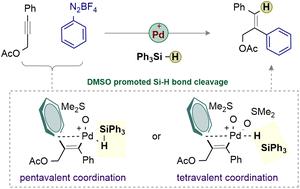
The regioselective hydro-functionalization reaction is a powerful method to convert readily available alkynes into structurally diverse olefins. Such an efficient syn-1,2-hydroarylation of yne-acetates is described herein using aryl diazonium salts and silanes as aryl and hydride sources, respectively. The transformation shows excellent functional group tolerance and applications to late-stage functionalization, providing straightforward access to trisubstituted allyl acetates. DFT analysis sheds light on the mechanism, particularly on the role of DMSO solvent in assisting the Si–H bond cleavage.
中文翻译:

内部炔烃的区域选择性 syn-1,2-氢芳基化
区域选择性氢功能化反应是将现成的炔烃转化为结构多样的烯烃的强大方法。本文分别使用芳基重氮盐和硅烷作为芳基和氢化物源描述了乙酸炔的这种高效的 syn-1,2-氢化芳基化反应。该转化显示出优异的官能团耐受性和应用于后期官能团化,为三取代烯丙基乙酸酯提供了直接的途径。DFT 分析阐明了该机制,特别是 DMSO 溶剂在协助 Si-H 键裂解中的作用。
更新日期:2024-10-23
中文翻译:

内部炔烃的区域选择性 syn-1,2-氢芳基化
区域选择性氢功能化反应是将现成的炔烃转化为结构多样的烯烃的强大方法。本文分别使用芳基重氮盐和硅烷作为芳基和氢化物源描述了乙酸炔的这种高效的 syn-1,2-氢化芳基化反应。该转化显示出优异的官能团耐受性和应用于后期官能团化,为三取代烯丙基乙酸酯提供了直接的途径。DFT 分析阐明了该机制,特别是 DMSO 溶剂在协助 Si-H 键裂解中的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号