Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RNA G-quadruplexes form scaffolds that promote neuropathological α-synuclein aggregation
Cell ( IF 45.5 ) Pub Date : 2024-10-18 , DOI: 10.1016/j.cell.2024.09.037 Kazuya Matsuo, Sefan Asamitsu, Kohei Maeda, Hiroyoshi Suzuki, Kosuke Kawakubo, Ginji Komiya, Kenta Kudo, Yusuke Sakai, Karin Hori, Susumu Ikenoshita, Shingo Usuki, Shiori Funahashi, Hideki Oizumi, Atsushi Takeda, Yasushi Kawata, Tomohiro Mizobata, Norifumi Shioda, Yasushi Yabuki
Cell ( IF 45.5 ) Pub Date : 2024-10-18 , DOI: 10.1016/j.cell.2024.09.037 Kazuya Matsuo, Sefan Asamitsu, Kohei Maeda, Hiroyoshi Suzuki, Kosuke Kawakubo, Ginji Komiya, Kenta Kudo, Yusuke Sakai, Karin Hori, Susumu Ikenoshita, Shingo Usuki, Shiori Funahashi, Hideki Oizumi, Atsushi Takeda, Yasushi Kawata, Tomohiro Mizobata, Norifumi Shioda, Yasushi Yabuki
|
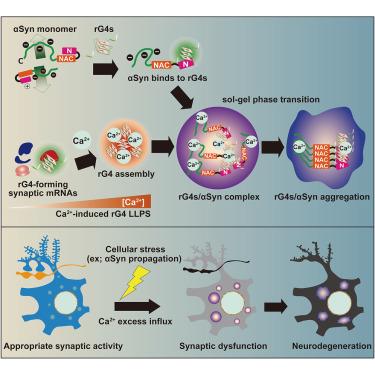
Synucleinopathies, including Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy, are triggered by α-synuclein aggregation, triggering progressive neurodegeneration. However, the intracellular α-synuclein aggregation mechanism remains unclear. Herein, we demonstrate that RNA G-quadruplex assembly forms scaffolds for α-synuclein aggregation, contributing to neurodegeneration. Purified α-synuclein binds RNA G-quadruplexes directly through the N terminus. RNA G-quadruplexes undergo Ca2+-induced phase separation and assembly, accelerating α-synuclein sol-gel phase transition. In α-synuclein preformed fibril-treated neurons, RNA G-quadruplex assembly comprising synaptic mRNAs co-aggregates with α-synuclein upon excess cytoplasmic Ca2+ influx, eliciting synaptic dysfunction. Forced RNA G-quadruplex assembly using an optogenetic approach evokes α-synuclein aggregation, causing neuronal dysfunction and neurodegeneration. The administration of 5-aminolevulinic acid, a protoporphyrin IX prodrug, prevents RNA G-quadruplex phase separation, thereby attenuating α-synuclein aggregation, neurodegeneration, and progressive motor deficits in α-synuclein preformed fibril-injected synucleinopathic mice. Therefore, Ca2+ influx-induced RNA G-quadruplex assembly accelerates α-synuclein phase transition and aggregation, potentially contributing to synucleinopathies.
中文翻译:

RNA G 四链体形成促进神经病理学 α-突触核蛋白聚集的支架
突触核蛋白病,包括帕金森病、路易体痴呆和多系统萎缩,是由 α-突触核蛋白聚集引发的,从而引发进行性神经变性。然而,细胞内 α-突触核蛋白聚集机制仍不清楚。在此,我们证明 RNA G -四链体组装形成 α-突触核蛋白聚集的支架,有助于神经退化。纯化的 α-突触核蛋白直接通过 N 末端结合 RNA G -四链体。RNA G-四链体经历 Ca2+ 诱导的相分离和组装,加速 α-突触核蛋白溶胶-凝胶相变。在 α-突触核蛋白预先形成的原纤维处理的神经元中,包含突触 mRNA 的 RNA G -四链体组装在细胞质 Ca2+ 过量流入时与 α-突触核蛋白共聚集,引发突触功能障碍。使用光遗传学方法的强制 RNA G 四链体组装会诱发 α-突触核蛋白聚集,导致神经元功能障碍和神经退化。5-氨基乙酰丙酸(一种原卟啉 IX 前药)的给药可防止 RNA G -四链体相分离,从而减弱 α-突触核蛋白预形成的突触核蛋白注射突触核蛋白小鼠的 α-突触核蛋白聚集、神经退行性和进行性运动缺陷。因此,Ca2+ 内流诱导的 RNA G-四链体组装加速了 α-突触核蛋白的相变和聚集,可能导致突触核蛋白病。
更新日期:2024-10-18
中文翻译:

RNA G 四链体形成促进神经病理学 α-突触核蛋白聚集的支架
突触核蛋白病,包括帕金森病、路易体痴呆和多系统萎缩,是由 α-突触核蛋白聚集引发的,从而引发进行性神经变性。然而,细胞内 α-突触核蛋白聚集机制仍不清楚。在此,我们证明 RNA G -四链体组装形成 α-突触核蛋白聚集的支架,有助于神经退化。纯化的 α-突触核蛋白直接通过 N 末端结合 RNA G -四链体。RNA G-四链体经历 Ca2+ 诱导的相分离和组装,加速 α-突触核蛋白溶胶-凝胶相变。在 α-突触核蛋白预先形成的原纤维处理的神经元中,包含突触 mRNA 的 RNA G -四链体组装在细胞质 Ca2+ 过量流入时与 α-突触核蛋白共聚集,引发突触功能障碍。使用光遗传学方法的强制 RNA G 四链体组装会诱发 α-突触核蛋白聚集,导致神经元功能障碍和神经退化。5-氨基乙酰丙酸(一种原卟啉 IX 前药)的给药可防止 RNA G -四链体相分离,从而减弱 α-突触核蛋白预形成的突触核蛋白注射突触核蛋白小鼠的 α-突触核蛋白聚集、神经退行性和进行性运动缺陷。因此,Ca2+ 内流诱导的 RNA G-四链体组装加速了 α-突触核蛋白的相变和聚集,可能导致突触核蛋白病。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号