Diabetologia ( IF 8.4 ) Pub Date : 2024-10-18 , DOI: 10.1007/s00125-024-06288-0 Sakina Ali, Xavier Vidal-Gómez, Megan Piquet, Luisa Vergori, Gilles Simard, Séverine Dubois, Pierre-Henri Ducluzeau, Pascal Pomiès, Sarah Kamli-Salino, Mirela Delibégovic, Samir Henni, Frédéric Gagnadoux, Ramaroson Andriantsitohaina, M. Carmen Martínez
|
Aims/hypothesis
Metabolic disorders associated with abdominal obesity, dyslipidaemia, arterial hypertension and hyperglycaemia are risk factors for the development of insulin resistance. Extracellular vesicles (EVs) may play an important role in the regulation of metabolic signalling pathways in insulin resistance and associated complications.
Methods
Circulating large EVs (lEVs) and small EVs (sEVs) from individuals with (IR group) and without insulin resistance (n-IR group) were isolated and characterised. lEVs and sEVs were administered by i.v. injection to mice and systemic, adipose tissue and liver insulin signalling were analysed. The role of phosphatases was analysed in target tissues and cells.
Results
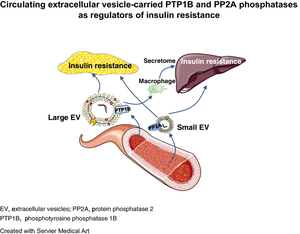
Injection of lEVs and sEVs from IR participants impaired systemic, adipose tissue and liver insulin signalling in mice, while EVs from n-IR participants had no effect. Moreover, lEVs and sEVs from IR participants brought about a twofold increase in adipocyte size and adipogenic gene expression. EVs from IR participants expressed two types of phosphatases, phosphotyrosine 1 phosphatase (PTP1B) and protein phosphatase 2 (PP2A), IR lEVs being enriched with the active form of PTP1B while IR sEVs mainly carried active PP2A. Blockade of PTP1B activity in IR lEVs fully restored IRS1 and Akt phosphorylation in adipocytes and blunted insulin-induced Akt phosphorylation by inhibition of the macrophage secretome in hepatocytes. Conversely, blockade of PP2A activity in IR sEVs completely prevented insulin resistance in adipocytes and hepatocytes.
Conclusions/interpretation
These data demonstrate that inhibition of phosphatases carried by EVs from IR participants rescues insulin signalling in adipocytes and hepatocytes and point towards PTP1B and PP2A carried by IR EVs as being novel potential therapeutic targets against insulin resistance in adipose tissue and liver and the development of obesity.
Graphical Abstract
中文翻译:

循环细胞外囊泡携带的 PTP1B 和 PP2A 磷酸酶作为胰岛素抵抗的调节剂
目标/假设
与腹部肥胖、血脂异常、动脉高血压和高血糖相关的代谢紊乱是胰岛素抵抗发展的危险因素。细胞外囊泡 (EVs) 可能在胰岛素抵抗和相关并发症中代谢信号通路的调节中发挥重要作用。
方法
分离和表征来自有 (IR 组) 和无胰岛素抵抗 (n-IR 组) 的个体的循环大型 EV (lEVs) 和小型 EVs (sEVs)。通过 i.v. 注射小鼠并分析全身、脂肪组织和肝脏胰岛素信号传导的 lEVs 和 sEVs。分析磷酸酶在靶组织和细胞中的作用。
结果
来自 IR 参与者的 lEVs 和 sEVs 注射损害了小鼠的全身、脂肪组织和肝脏胰岛素信号传导,而来自 n-IR 参与者的 EV 没有影响。此外,来自 IR 参与者的 lEVs 和 sEVs 使脂肪细胞大小和成脂基因表达增加了两倍。来自 IR 参与者的 EV 表达两种类型的磷酸酶,磷酸酪氨酸 1 磷酸酶 (PTP1B) 和蛋白质磷酸酶 2 (PP2A),IR lEVs 富含 PTP1B 的活性形式,而 IR sEVs 主要携带活性 PP2A。阻断 IR lEV 中 PTP1B 活性完全恢复了脂肪细胞中的 IRS1 和 Akt 磷酸化,并通过抑制肝细胞中巨噬细胞分泌组减弱了胰岛素诱导的 Akt 磷酸化。相反,阻断 IR sEVs 中的 PP2A 活性完全阻止了脂肪细胞和肝细胞中的胰岛素抵抗。
结论/解释
这些数据表明,抑制 IR 参与者的 EV 携带的磷酸酶可以挽救脂肪细胞和肝细胞中的胰岛素信号传导,并指出 IR EV 携带的 PTP1B 和 PP2A 是针对脂肪组织和肝脏中胰岛素抵抗和肥胖发展的新型潜在治疗靶点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号