当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scale-Up Preparation of Best-In-Class Orally Bioavailable CXCR4 Antagonist EMU-116 in an Academic Setting
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-18 , DOI: 10.1021/acs.oprd.4c00246 Leon Jacobs, Eric J. Miller, Robert J. Wilson, Edgars Jecs, Paul Joseph Tholath, Huy H. Nguyen, Manohar T. Saindane, Yesim Altas-Tahirovic, Lawrence J. Wilson, Dennis C. Liotta
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-10-18 , DOI: 10.1021/acs.oprd.4c00246 Leon Jacobs, Eric J. Miller, Robert J. Wilson, Edgars Jecs, Paul Joseph Tholath, Huy H. Nguyen, Manohar T. Saindane, Yesim Altas-Tahirovic, Lawrence J. Wilson, Dennis C. Liotta
|
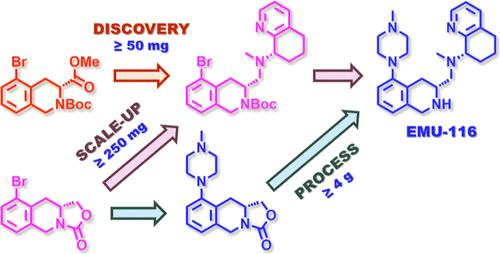
CXCR4 is a seven-transmembrane chemokine receptor that is intimately involved in stem cell niche maintenance and immune cell trafficking. Among several other pathophysiological states for which CXCR4 mis regulation is implicated, various hematological malignancies and solid tumors hijack this chemokine network by dramatically overexpressing CXCR4 and its cognate chemokine ligand CXCL12. Upregulation of the CXCR4/CXCL12 axis in cancer drives tumor progression through several mechanisms, which makes CXCR4 a promising target for the development of anticancer therapeutics. Herein, we report the preparative scale synthesis of a novel, best-in-class, orally bioavailable small molecule CXCR4 antagonist, EMU-116. Two synthetic strategies for production of EMU-116 were pursued. While the first discovery-focused synthesis facilitated late-stage diversification to drive structure–activity relationship determinations, the second process-focused synthesis delivered EMU-116 more efficiently in higher overall yield with enhanced stereocontrol. For both synthetic routes, Buchwald–Hartwig amination of key aryl bromide intermediates enabled installation of the N-methylpiperazine appendage of EMU-116. Synthetic methods devised to prepare (R)-9-bromo-1,5,10,10a-tetrahydro-3H-oxazolo[3,4-b]isoquinolin-3-one, the key aryl bromide intermediate required for the process-focused synthesis, are reported. In addition, an improved preparative method of known synthon (S)–N-methyl-5,6,7,8-tetrahydroquinolin-8-amine is highlighted by elevated overall yield, enhanced diastereoselectivity, and robust purification by crystallization. Further elaboration of these two intermediates, coupling via reductive amination to furnish the full EMU-116 scaffold, removal of protecting groups, and final product purification techniques are also reported. Overall, the synthetic methods described herein enabled reliable and efficient production of multigram quantities of EMU-116 and are anticipated to be amenable to larger scale production.
中文翻译:

在学术环境中同类最佳的口服生物可利用 CXCR4 拮抗剂 EMU-116 的放大制备
CXCR4 是一种七次跨膜趋化因子受体,与干细胞生态位维持和免疫细胞运输密切相关。在涉及 CXCR4 错误调节的其他几种病理生理状态中,各种血液系统恶性肿瘤和实体瘤通过显着过表达 CXCR4 及其同源趋化因子配体 CXCL12 劫持了这个趋化因子网络。癌症中 CXCR4/CXCL12 轴的上调通过多种机制驱动肿瘤进展,这使得 CXCR4 成为抗癌疗法开发的有前途的靶点。在此,我们报道了一种新的、一流的、口服生物可利用的小分子 CXCR4 拮抗剂 EMU-116 的制备规模合成。追求两种生产 EMU-116 的合成策略。第一次以发现为重点的合成促进了后期多样化以驱动结构-活性关系的确定,而第二次以过程为中心的合成以更高的总产量和增强的立体控制更有效地递送了 EMU-116。对于这两种合成路线,关键芳基溴中间体的 Buchwald-Hartwig 胺化反应能够安装 EMU-116 的 N-甲基哌嗪附属物。报道了制备 (R)-9-溴-1,5,10,10a-四氢-3H-恶唑并[3,4-b]异喹啉-3-酮的合成方法,这是以工艺为重点的合成所需的关键芳基溴中间体。此外,已知合成子 (S)-N-甲基-5,6,7,8-四氢喹啉-8-胺的改进制备方法的特点是总产率更高、非对映选择性增强和结晶稳定纯化。 还报道了这两种中间体的进一步阐述,通过还原胺化偶联以提供完整的 EMU-116 支架,去除保护基团以及最终产品纯化技术。总体而言,本文描述的合成方法能够可靠和高效地生产数克级的 EMU-116,并且有望适应更大规模的生产。
更新日期:2024-10-18
中文翻译:

在学术环境中同类最佳的口服生物可利用 CXCR4 拮抗剂 EMU-116 的放大制备
CXCR4 是一种七次跨膜趋化因子受体,与干细胞生态位维持和免疫细胞运输密切相关。在涉及 CXCR4 错误调节的其他几种病理生理状态中,各种血液系统恶性肿瘤和实体瘤通过显着过表达 CXCR4 及其同源趋化因子配体 CXCL12 劫持了这个趋化因子网络。癌症中 CXCR4/CXCL12 轴的上调通过多种机制驱动肿瘤进展,这使得 CXCR4 成为抗癌疗法开发的有前途的靶点。在此,我们报道了一种新的、一流的、口服生物可利用的小分子 CXCR4 拮抗剂 EMU-116 的制备规模合成。追求两种生产 EMU-116 的合成策略。第一次以发现为重点的合成促进了后期多样化以驱动结构-活性关系的确定,而第二次以过程为中心的合成以更高的总产量和增强的立体控制更有效地递送了 EMU-116。对于这两种合成路线,关键芳基溴中间体的 Buchwald-Hartwig 胺化反应能够安装 EMU-116 的 N-甲基哌嗪附属物。报道了制备 (R)-9-溴-1,5,10,10a-四氢-3H-恶唑并[3,4-b]异喹啉-3-酮的合成方法,这是以工艺为重点的合成所需的关键芳基溴中间体。此外,已知合成子 (S)-N-甲基-5,6,7,8-四氢喹啉-8-胺的改进制备方法的特点是总产率更高、非对映选择性增强和结晶稳定纯化。 还报道了这两种中间体的进一步阐述,通过还原胺化偶联以提供完整的 EMU-116 支架,去除保护基团以及最终产品纯化技术。总体而言,本文描述的合成方法能够可靠和高效地生产数克级的 EMU-116,并且有望适应更大规模的生产。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号