Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically driven nonheme iron complex-catalyzed oxidation reactions using water as an oxygen source
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-16 , DOI: 10.1016/j.jcat.2024.115792 Songgang Huang, Yan Wang, Si Si, Mei Yan, Weimin Zhang, Wenhua Ji, Jie Chen, Wonwoo Nam, Bin Wang
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-16 , DOI: 10.1016/j.jcat.2024.115792 Songgang Huang, Yan Wang, Si Si, Mei Yan, Weimin Zhang, Wenhua Ji, Jie Chen, Wonwoo Nam, Bin Wang
|
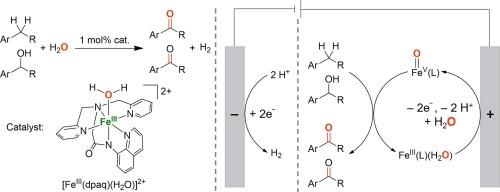
High-valent metal-oxo species have been invoked as key intermediates in enzymatic and biomimetic oxidation reactions. The generation of high-valent metal-oxo species using water (H2 O) as an oxygen source represents one of the most environmentally friendly approaches in developing biologically inspired oxidation catalysis. Herein, we report the electrochemical oxidation of benzylic C−H bonds and alcohols utilizing a mononuclear nonheme iron(III)-monoamidate complex [FeIII (dpaq)(H2 O)]2+ (dpaq = 2-[bis(pyridin-2-ylmethyl)]amino-N -quinolin-8-yl-acetamidate) as a catalyst and H2 O as an oxygen source. Selective benzylic C−H bond oxidation of alkanes to ketones was achieved in 43–85 % yields, and primary and secondary alcohols were converted to the corresponding aldehydes and ketones, respectively, in 46–95 % yields. The generation of an iron(V)-oxo species [FeV (O)(dpaq)]2+ from proton-coupled electron-transfer (PCET) oxidation of the iron(III) aqua complex [FeIII (dpaq)(H2 O)]2+ was evidenced by cyclic voltammetry analysis; the iron(V)-oxo species [FeV (O)(dpaq)]2+ was recently detected using transient absorption spectroscopy in water oxidation reactions. Mechanistic studies revealed that electrochemical oxidation of alcohols catalyzed by FeIII (dpaq) is a two-electron oxidation process, hydrogen-atom transfer (HAT) from the α-C−H bond of alcohols by iron(V)-oxo species is the rate-determining step, and there is a remarkable charge transfer from the highly electrophilic iron(V)-oxo species to the alcohols in the HAT step. This research paves a significant groundwork aimed at developing electrochemically driven biomimetic asymmetric oxidation reactions catalyzed by nonheme metal complexes supported by chiral ligands.
中文翻译:

以水为氧源的电化学驱动的非血红素铁络合物催化的氧化反应
高价金属羰基合成物质已被用作酶促和仿生氧化反应的关键中间体。使用水 (H2O) 作为氧源生成高价金属氧代物质是开发生物启发氧化催化的最环保方法之一。在此,我们报道了利用单核非血红素铁 (III) -单酰胺酸盐络合物 [FeIII(dpaq)(H2O)]2+ (dpaq = 2-[bis(pyridin-2-ylmethyl)]氨基-N-喹啉-8-基乙酰胺酸盐)作为催化剂和 H2O 作为氧源。烷烃选择性地苄基 C-H 键氧化为酮,产率为 43-85%,伯醇和仲醇分别转化为相应的醛和酮,产率为 46-95%。循环伏安法分析证明了铁 (III) 水络合物 [FeIII(dpaq)(H2O)]2+ 的质子耦合电子转移 (PCET) 氧化生成铁 (V)-氧代物质 [FeV(O)(dpaq)]2+;最近在水氧化反应中使用瞬态吸收光谱法检测了铁 (V)-氧代物质 [FeV(O)(dpaq)]2+。机理研究表明,FeIII (dpaq) 催化的醇的电化学氧化是一个双电子氧化过程,铁 (V)-氧代物质从醇的 α-C-H 键进行氢原子转移 (HAT) 是速率决定步骤,并且在 HAT 步骤中,从高亲电性铁 (V)-氧代物质到醇的电荷转移显着。这项研究为开发由手性配体支持的非血红素金属络合物催化的电化学驱动的仿生不对称氧化反应奠定了重要的基础。
更新日期:2024-10-16
中文翻译:

以水为氧源的电化学驱动的非血红素铁络合物催化的氧化反应
高价金属羰基合成物质已被用作酶促和仿生氧化反应的关键中间体。使用水 (H2O) 作为氧源生成高价金属氧代物质是开发生物启发氧化催化的最环保方法之一。在此,我们报道了利用单核非血红素铁 (III) -单酰胺酸盐络合物 [FeIII(dpaq)(H2O)]2+ (dpaq = 2-[bis(pyridin-2-ylmethyl)]氨基-N-喹啉-8-基乙酰胺酸盐)作为催化剂和 H2O 作为氧源。烷烃选择性地苄基 C-H 键氧化为酮,产率为 43-85%,伯醇和仲醇分别转化为相应的醛和酮,产率为 46-95%。循环伏安法分析证明了铁 (III) 水络合物 [FeIII(dpaq)(H2O)]2+ 的质子耦合电子转移 (PCET) 氧化生成铁 (V)-氧代物质 [FeV(O)(dpaq)]2+;最近在水氧化反应中使用瞬态吸收光谱法检测了铁 (V)-氧代物质 [FeV(O)(dpaq)]2+。机理研究表明,FeIII (dpaq) 催化的醇的电化学氧化是一个双电子氧化过程,铁 (V)-氧代物质从醇的 α-C-H 键进行氢原子转移 (HAT) 是速率决定步骤,并且在 HAT 步骤中,从高亲电性铁 (V)-氧代物质到醇的电荷转移显着。这项研究为开发由手性配体支持的非血红素金属络合物催化的电化学驱动的仿生不对称氧化反应奠定了重要的基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号